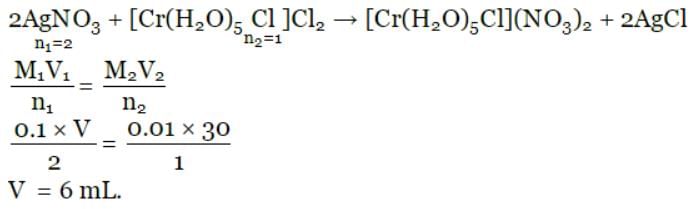All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Coordination Chemistry (d block) for Chemistry Exam
Which of the following cannot act as a ligand:- a)AsH3
- b)NO+
- c)BF3
- d)Cl–
Correct answer is option 'C'. Can you explain this answer?
Which of the following cannot act as a ligand:
a)
AsH3
b)
NO+
c)
BF3
d)
Cl–
|
|
Pooja Choudhury answered |
- NH3 is a neutral ligand.
- NO+ is a cationic ligand.
- Cl– is an anionic ligand.
- However, BF3 is not a ligand as it does not have lone pairs to donate and can not share a pair of electrons.
The zero magnetic moment of octahedral  is due to:a) Low spin
is due to:a) Low spin  Ni(IV) complexb) Low spin
Ni(IV) complexb) Low spin  Ni(II) complexc) High spin
Ni(II) complexc) High spin  Ni(II) complexd) High spin
Ni(II) complexd) High spin  Ni(IV) complex The correct answer is option 'A'. Can you explain this answer?
Ni(IV) complex The correct answer is option 'A'. Can you explain this answer?
|
|
Pooja Choudhury answered |
Oxidation state of Ni in [NiF6]2- :
x − 6 = −2
x = + 4
In +4 oxidation state, fluoride also behaves as a strong field ligand.
⇒ low spin d6 Ni(IV) complex.
⇒ unpaired electrons = 0.
⇒ zero magnetic moment.
x − 6 = −2
x = + 4
In +4 oxidation state, fluoride also behaves as a strong field ligand.
⇒ low spin d6 Ni(IV) complex.
⇒ unpaired electrons = 0.
⇒ zero magnetic moment.
As a ligand Cl– is:
- a)Only a σ–donor
- b)Both a σ–donor and a π–donor
- c)Only a π–donor
- d)A σ–donor and a σ–acceptor
Correct answer is option 'B'. Can you explain this answer?
As a ligand Cl– is:
a)
Only a σ–donor
b)
Both a σ–donor and a π–donor
c)
Only a π–donor
d)
A σ–donor and a σ–acceptor
|
|
Vikram Kapoor answered |
Cl- have extra e and also empty orbital so act as sigma as well as pi donor..NH3 is only sigma donor bcoz of unavailbility of vacant orbital.
The crystal field stabilization energy (CFSE), will be the highest for:- a)[CoF6]3-
- b)[Co(CNS)4]2-
- c)[Mn(H2O)6]2+
- d)[Co(NH3)6]3+
Correct answer is option 'D'. Can you explain this answer?
The crystal field stabilization energy (CFSE), will be the highest for:
a)
[CoF6]3-
b)
[Co(CNS)4]2-
c)
[Mn(H2O)6]2+
d)
[Co(NH3)6]3+

|
Kaavya Sengupta answered |
The crystal field stabilization energy (CFSE) is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. Conversely, the eg orbitals (in the octahedral case) are higher in energy than in the barycenter, so putting electrons in these reduces the amount of CFSE.
Which of the following is a spin paired complex ion?- a)[Cr(NH3)6]3+
- b)[Cr(CN)6]3-
- c)[Fe(C2O4)3]3-
- d)[Co(NH3)6]3+
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a spin paired complex ion?
a)
[Cr(NH3)6]3+
b)
[Cr(CN)6]3-
c)
[Fe(C2O4)3]3-
d)
[Co(NH3)6]3+

|
Saikat Ghoshal answered |
Spin paired complex ions are those in which all the electrons are paired up in the same direction, resulting in a diamagnetic complex. Diamagnetic complexes are not attracted to a magnetic field and have a low magnetic moment.
The correct answer is option 'D', [Co(NH3)6]3.
Explanation:
- Coordination number of complex ion = 6
- Oxidation state of central metal ion = +3
- Cobalt has 27 electrons, so [Co(NH3)6]3+ has 24 electrons
- NH3 is a strong field ligand, so it will pair up the electrons in the t2g orbitals before occupying the eg orbitals
- Therefore, all the electrons in the complex ion are paired up in the same direction, resulting in a diamagnetic complex
- Hence, [Co(NH3)6]3+ is a spin paired complex ion
Let's look at the other options:
a) [Cr(NH3)6]3+: Chromium has a d4 configuration, so it has one unpaired electron in the t2g orbitals. Hence, it is a paramagnetic complex.
b) [Cr(CN)6]3-: Cyanide is a strong field ligand, so it will pair up the electrons in the t2g orbitals before occupying the eg orbitals. However, chromium has a d3 configuration, so it will have one unpaired electron in the eg orbitals. Hence, it is a paramagnetic complex.
c) [Fe(C2O4)3]3-: Iron has a d5 configuration, so it has one unpaired electron in the t2g orbitals. Hence, it is a paramagnetic complex.
The correct answer is option 'D', [Co(NH3)6]3.
Explanation:
- Coordination number of complex ion = 6
- Oxidation state of central metal ion = +3
- Cobalt has 27 electrons, so [Co(NH3)6]3+ has 24 electrons
- NH3 is a strong field ligand, so it will pair up the electrons in the t2g orbitals before occupying the eg orbitals
- Therefore, all the electrons in the complex ion are paired up in the same direction, resulting in a diamagnetic complex
- Hence, [Co(NH3)6]3+ is a spin paired complex ion
Let's look at the other options:
a) [Cr(NH3)6]3+: Chromium has a d4 configuration, so it has one unpaired electron in the t2g orbitals. Hence, it is a paramagnetic complex.
b) [Cr(CN)6]3-: Cyanide is a strong field ligand, so it will pair up the electrons in the t2g orbitals before occupying the eg orbitals. However, chromium has a d3 configuration, so it will have one unpaired electron in the eg orbitals. Hence, it is a paramagnetic complex.
c) [Fe(C2O4)3]3-: Iron has a d5 configuration, so it has one unpaired electron in the t2g orbitals. Hence, it is a paramagnetic complex.
Hybridizat ion of Ni(II) in K2[NiBr4] is:- a)dsp2
- b)sp3
- c)sp2d
- d)d3s
Correct answer is option 'B'. Can you explain this answer?
Hybridizat ion of Ni(II) in K2[NiBr4] is:
a)
dsp2
b)
sp3
c)
sp2d
d)
d3s

|
Asf Institute answered |
- In K2[NiBr4], the nickel ion is surrounded by four bromide ions.
- The electronic configuration of Ni(II) is 3d8 4s2.
- To form the complex, the nickel ion utilizes one 4s and three 4p orbitals to hybridize into four sp3 hybrid orbitals.
- The hybrid orbitals are then used to form sigma bonds with the four bromide ions.
- Therefore, the hybridization of Ni(II) in K2[NiBr4] is sp3.
- The electronic configuration of Ni(II) is 3d8 4s2.
- To form the complex, the nickel ion utilizes one 4s and three 4p orbitals to hybridize into four sp3 hybrid orbitals.
- The hybrid orbitals are then used to form sigma bonds with the four bromide ions.
- Therefore, the hybridization of Ni(II) in K2[NiBr4] is sp3.
Which statement(s) is (are) true:(I) High spin complexes are always paramagnetic(II) Low spin complexes are always diamagnetic(III)  is more likely to form a low spin complex than
is more likely to form a low spin complex than  (IV) Tetrahedral complexes are more likely to be low spin than high spin
(IV) Tetrahedral complexes are more likely to be low spin than high spin
a)I, II, and IVb)I onlyc)I, and IId)I, II, and IIIThe correct answer is option 'C'. Can you explain this answer?

|
Sayantan answered |
Though none is the option is fully crrct...but u can say 1 nd 2 is partially crrt
The first row transition metal complexes having tetrahedral geometry are high–spin due to:- a)Δt > P
- b)Δt < P
- c)Δt = P
- d)Δt > Δ0
Correct answer is option 'B'. Can you explain this answer?
The first row transition metal complexes having tetrahedral geometry are high–spin due to:
a)
Δt > P
b)
Δt < P
c)
Δt = P
d)
Δt > Δ0
|
|
Alka Sahu answered |
Because pairing energy is more that means electrons are not get paired against the Hund' s rule
The magnetic moment of the complex K3[CoF6] is 5.0 μB . The total stabilization energy will be:- a)-0.4 Δ0
- b)-0.4 Δ0 + P
- c)-2.4 Δ0 + 3P
- d)-1.8 Δ0 + 3P
Correct answer is option 'A'. Can you explain this answer?
The magnetic moment of the complex K3[CoF6] is 5.0 μB . The total stabilization energy will be:
a)
-0.4 Δ0
b)
-0.4 Δ0 + P
c)
-2.4 Δ0 + 3P
d)
-1.8 Δ0 + 3P

|
Asf Institute answered |
Magnetic moment of K3[CoF6]
- The magnetic moment of the complex K3[CoF6] is 5.0 B.
Stabilization energy
- The stabilization energy is the energy released during the formation of a complex from its constituent ions.
- The greater the stabilization energy, the more stable the complex.
- The stabilization energy can be calculated using the crystal field theory.
Crystal field theory
- According to the crystal field theory, the metal ion in a complex is surrounded by a set of negatively charged ligands.
- The ligands repel the electrons in the metal ion, causing the energy levels to split.
- The magnitude of the splitting depends on the nature of the ligands and the geometry of the complex.
- The energy difference between the highest and lowest energy levels determines the magnetic properties of the complex.
- The magnetic moment of the complex is related to the number of unpaired electrons in the highest energy level.
Calculation of stabilization energy
- The stabilization energy can be calculated using the following equation:
ΔE = -0.4Bn(unpaired)
where ΔE is the stabilization energy, B is the crystal field splitting energy, n is the number of ligands, and unpaired is the number of unpaired electrons in the highest energy level.
- In this case, the complex has a magnetic moment of 5.0 B, which indicates the presence of one unpaired electron in the highest energy level.
- Therefore, unpaired = 1 and n = 6 (since there are six ligands in the complex).
- The crystal field splitting energy for octahedral complexes with strong field ligands is 0.4 Δo, where Δo is the crystal field splitting parameter.
- For Co(III), Δo is approximately 10,000 cm-1.
- Therefore, B = 0.4 x 10,000 = 4000 cm-1.
- Substituting these values into the equation, we get:
ΔE = -0.4 x 4000 x 1 x 6 = -9600 cm-1 = -0.96 eV.
Conclusion
- The total stabilization energy of the complex K3[CoF6] is -0.96 eV or -9600 cm-1.
- The correct answer is option 'A'.
- The magnetic moment of the complex K3[CoF6] is 5.0 B.
Stabilization energy
- The stabilization energy is the energy released during the formation of a complex from its constituent ions.
- The greater the stabilization energy, the more stable the complex.
- The stabilization energy can be calculated using the crystal field theory.
Crystal field theory
- According to the crystal field theory, the metal ion in a complex is surrounded by a set of negatively charged ligands.
- The ligands repel the electrons in the metal ion, causing the energy levels to split.
- The magnitude of the splitting depends on the nature of the ligands and the geometry of the complex.
- The energy difference between the highest and lowest energy levels determines the magnetic properties of the complex.
- The magnetic moment of the complex is related to the number of unpaired electrons in the highest energy level.
Calculation of stabilization energy
- The stabilization energy can be calculated using the following equation:
ΔE = -0.4Bn(unpaired)
where ΔE is the stabilization energy, B is the crystal field splitting energy, n is the number of ligands, and unpaired is the number of unpaired electrons in the highest energy level.
- In this case, the complex has a magnetic moment of 5.0 B, which indicates the presence of one unpaired electron in the highest energy level.
- Therefore, unpaired = 1 and n = 6 (since there are six ligands in the complex).
- The crystal field splitting energy for octahedral complexes with strong field ligands is 0.4 Δo, where Δo is the crystal field splitting parameter.
- For Co(III), Δo is approximately 10,000 cm-1.
- Therefore, B = 0.4 x 10,000 = 4000 cm-1.
- Substituting these values into the equation, we get:
ΔE = -0.4 x 4000 x 1 x 6 = -9600 cm-1 = -0.96 eV.
Conclusion
- The total stabilization energy of the complex K3[CoF6] is -0.96 eV or -9600 cm-1.
- The correct answer is option 'A'.
The complex with maximum CFSE is:- a)[CoCl4]2–
- b)[Co(H2O)6]3+
- c)[CoF6]3–
- d)[CoF3(H2O)3]
Correct answer is option 'B'. Can you explain this answer?
The complex with maximum CFSE is:
a)
[CoCl4]2–
b)
[Co(H2O)6]3+
c)
[CoF6]3–
d)
[CoF3(H2O)3]
|
|
Pooja Choudhury answered |
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis,
Fe(CO)5,Ni(CO)4 etc.
In the complexes [Fe(CN)6]3– and [Co(en)3]3+, the coordination number of iron and cobalt are respectively (en = ethylenediamine):- a)6 and 6
- b)12 and 6
- c)6 and 3
- d)3 and 3
Correct answer is option 'A'. Can you explain this answer?
In the complexes [Fe(CN)6]3– and [Co(en)3]3+, the coordination number of iron and cobalt are respectively (en = ethylenediamine):
a)
6 and 6
b)
12 and 6
c)
6 and 3
d)
3 and 3
|
|
Vedika Singh answered |
- In 1st compound, CN is a unidentate ligand and hence it will surround the Fe atom from 6 different sides.
- On the other hand, the 2nd compound contains bidentate ligand so each (en) will have two different sites of donating electrons and hence it required 3 (en) atoms for surrounding Co atom from 6 different sides.
Hence A is correct.
The spinels CoFe2O4 and FeFe2O4, respectively are:
- a)Inverse and normal
- b)Inverse and inverse
- c)Normal and normal
- d)Normal and inverse
Correct answer is option 'A'. Can you explain this answer?
The spinels CoFe2O4 and FeFe2O4, respectively are:
a)
Inverse and normal
b)
Inverse and inverse
c)
Normal and normal
d)
Normal and inverse

|
Asf Institute answered |
- Spinels have a general formula AB2O4 and are classified as either normal or inverse.
- In a normal spinel, A2+ ions occupy the tetrahedral sites, and B3+ ions are in octahedral sites.
- In an inverse spinel, half of the B3+ ions and all A2+ ions occupy octahedral sites, while the remaining B3+ ions occupy tetrahedral sites.
- CoFe2O4 is inverse because Co2+ prefers octahedral sites.
- FeFe2O4 (magnetite) is also inverse with Fe3+ ions distributed as described.
The CFSE for the following d3 metal ions (V2+, Cr3+, Mo3+) decrease in the following order :- a)V2+ > Cr3+ > Mo3+
- b)Cr3+ > V2+ > Mo3+
- c)Mo3+ > Cr3+ > V2+
- d)Cr3+ > Mo3+ > V2+
Correct answer is option 'C'. Can you explain this answer?
The CFSE for the following d3 metal ions (V2+, Cr3+, Mo3+) decrease in the following order :
a)
V2+ > Cr3+ > Mo3+
b)
Cr3+ > V2+ > Mo3+
c)
Mo3+ > Cr3+ > V2+
d)
Cr3+ > Mo3+ > V2+
|
|
Pooja Choudhury answered |
Ti4+ is most stable as it has completely filled 3p orbital.
The octahedral complex of Cr (III) will be:a) in case of weak field ligand.b)
in case of weak field ligand.b) in case of the strong ligand.c)
in case of the strong ligand.c) always.d)
always.d) always.The correct answer is option 'C'. Can you explain this answer?
always.The correct answer is option 'C'. Can you explain this answer?

|
Sayantan answered |
Cr(lll) is a d3 system so there are two (n-1)d orbital is availble for hybdsn so it always show d2sp3 hybsn
The colour of potassium dichromate is due to:- a)d–d transition
- b)Transition in K+ ion.
- c)Ligand to metal charge transfer
- d)Metal-to-ligand charge transfer
Correct answer is option 'C'. Can you explain this answer?
The colour of potassium dichromate is due to:
a)
d–d transition
b)
Transition in K+ ion.
c)
Ligand to metal charge transfer
d)
Metal-to-ligand charge transfer

|
Edurev.iitjam answered |
Explanation:
The colour of potassium dichromate is due to Ligand to metal charge transfer.
- Potassium dichromate (K2Cr2O7) is an inorganic compound that contains the dichromate ion (Cr2O7 2-) as a ligand and potassium ions (K+) as the metal.
- When light interacts with a compound, it can be absorbed by the electrons present in the compound's atoms or ions.
- In the case of potassium dichromate, the dichromate ion (Cr2O7 2-) acts as a ligand and donates a pair of electrons to the central chromium ion (Cr).
- The dichromate ion has a deep orange color due to the presence of multiple double bonds between the oxygen and chromium atoms.
- When light passes through potassium dichromate, the electrons in the oxygen atoms of the dichromate ion absorb certain wavelengths of light.
- These absorbed wavelengths correspond to the complementary color of orange, which is blue.
- As a result, the transmitted light appears blue, giving potassium dichromate its characteristic color.
To summarize:
- The colour of potassium dichromate is due to ligand to metal charge transfer.
- The dichromate ion acts as a ligand and donates electrons to the central chromium ion.
- The absorption of specific wavelengths of light by the ligand-metal complex leads to the observed color of potassium dichromate.
The colour of potassium dichromate is due to Ligand to metal charge transfer.
- Potassium dichromate (K2Cr2O7) is an inorganic compound that contains the dichromate ion (Cr2O7 2-) as a ligand and potassium ions (K+) as the metal.
- When light interacts with a compound, it can be absorbed by the electrons present in the compound's atoms or ions.
- In the case of potassium dichromate, the dichromate ion (Cr2O7 2-) acts as a ligand and donates a pair of electrons to the central chromium ion (Cr).
- The dichromate ion has a deep orange color due to the presence of multiple double bonds between the oxygen and chromium atoms.
- When light passes through potassium dichromate, the electrons in the oxygen atoms of the dichromate ion absorb certain wavelengths of light.
- These absorbed wavelengths correspond to the complementary color of orange, which is blue.
- As a result, the transmitted light appears blue, giving potassium dichromate its characteristic color.
To summarize:
- The colour of potassium dichromate is due to ligand to metal charge transfer.
- The dichromate ion acts as a ligand and donates electrons to the central chromium ion.
- The absorption of specific wavelengths of light by the ligand-metal complex leads to the observed color of potassium dichromate.
The electronic configurations that have orbital angular momentum contribution in octahedral environment are:
- a)d1 and high spin d4
- b)d1 and d2
- c)d2 and high spin d5
- d)high spin d4 and high spin d6
Correct answer is option 'B'. Can you explain this answer?
The electronic configurations that have orbital angular momentum contribution in octahedral environment are:
a)
d1 and high spin d4
b)
d1 and d2
c)
d2 and high spin d5
d)
high spin d4 and high spin d6

|
Edurev.iitjam answered |
Electronic Configurations with Orbital Angular Momentum Contribution in Octahedral Environment
In an octahedral environment, the d-orbitals split into two sets of different energies due to the crystal field effect. The lower energy set, called the t2g set, consists of three orbitals (dxy, dyz, and dxz), while the higher energy set, called the eg set, consists of two orbitals (dx2-y2 and dz2).
The electronic configurations that have orbital angular momentum contribution in an octahedral environment are d1 and d2. Let's understand why:
A. d1 and high spin d4:
- In an octahedral environment, a d1 configuration means that only one electron occupies the t2g set.
- The electron occupies one of the three t2g orbitals (dxy, dyz, or dxz).
- Since there is only one electron, there is no orbital angular momentum contribution.
B. d1 and d2:
- In an octahedral environment, a d2 configuration means that two electrons occupy the t2g set.
- The two electrons can occupy any two of the three t2g orbitals (dxy, dyz, or dxz).
- The presence of two electrons in the t2g set introduces orbital angular momentum contribution.
- The two electrons have opposite spins, resulting in a net orbital angular momentum.
C. d2 and high spin d5:
- In an octahedral environment, a d2 configuration means that two electrons occupy the t2g set.
- The two electrons can occupy any two of the three t2g orbitals (dxy, dyz, or dxz).
- The presence of two electrons in the t2g set introduces orbital angular momentum contribution.
- The two electrons have opposite spins, resulting in a net orbital angular momentum.
- A d5 configuration means that five electrons occupy the t2g and eg sets.
- The five electrons can occupy any combination of the t2g and eg orbitals.
- However, the presence of five electrons in the d orbitals does not introduce additional orbital angular momentum contribution.
D. high spin d4 and high spin d6:
- A high spin d4 configuration means that four electrons occupy the t2g and eg sets.
- The four electrons can occupy any combination of the t2g and eg orbitals.
- However, the presence of four electrons in the d orbitals does not introduce additional orbital angular momentum contribution.
- Similarly, a high spin d6 configuration means that six electrons occupy the t2g and eg sets, but it also does not introduce additional orbital angular momentum contribution.
Therefore, the only electronic configurations that have orbital angular momentum contribution in an octahedral environment are d1 and d2, making option B the correct answer.
In an octahedral environment, the d-orbitals split into two sets of different energies due to the crystal field effect. The lower energy set, called the t2g set, consists of three orbitals (dxy, dyz, and dxz), while the higher energy set, called the eg set, consists of two orbitals (dx2-y2 and dz2).
The electronic configurations that have orbital angular momentum contribution in an octahedral environment are d1 and d2. Let's understand why:
A. d1 and high spin d4:
- In an octahedral environment, a d1 configuration means that only one electron occupies the t2g set.
- The electron occupies one of the three t2g orbitals (dxy, dyz, or dxz).
- Since there is only one electron, there is no orbital angular momentum contribution.
B. d1 and d2:
- In an octahedral environment, a d2 configuration means that two electrons occupy the t2g set.
- The two electrons can occupy any two of the three t2g orbitals (dxy, dyz, or dxz).
- The presence of two electrons in the t2g set introduces orbital angular momentum contribution.
- The two electrons have opposite spins, resulting in a net orbital angular momentum.
C. d2 and high spin d5:
- In an octahedral environment, a d2 configuration means that two electrons occupy the t2g set.
- The two electrons can occupy any two of the three t2g orbitals (dxy, dyz, or dxz).
- The presence of two electrons in the t2g set introduces orbital angular momentum contribution.
- The two electrons have opposite spins, resulting in a net orbital angular momentum.
- A d5 configuration means that five electrons occupy the t2g and eg sets.
- The five electrons can occupy any combination of the t2g and eg orbitals.
- However, the presence of five electrons in the d orbitals does not introduce additional orbital angular momentum contribution.
D. high spin d4 and high spin d6:
- A high spin d4 configuration means that four electrons occupy the t2g and eg sets.
- The four electrons can occupy any combination of the t2g and eg orbitals.
- However, the presence of four electrons in the d orbitals does not introduce additional orbital angular momentum contribution.
- Similarly, a high spin d6 configuration means that six electrons occupy the t2g and eg sets, but it also does not introduce additional orbital angular momentum contribution.
Therefore, the only electronic configurations that have orbital angular momentum contribution in an octahedral environment are d1 and d2, making option B the correct answer.
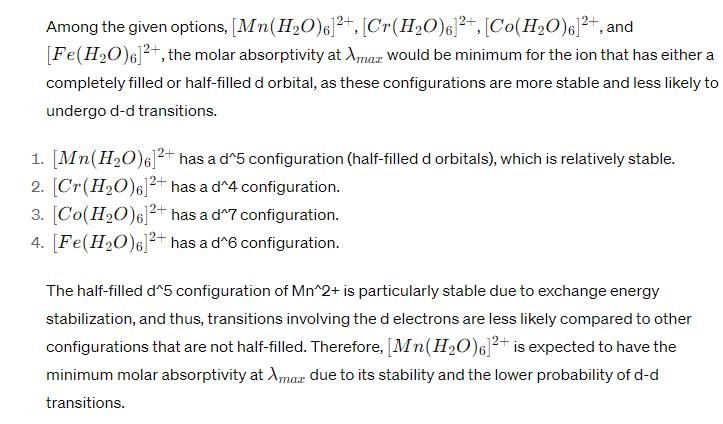
The molar absorptivity at λmax is minimum for:- a)[Mn (H2O)6]2+
- b)[Cr(H2O)6]2+
- c)[Co(H2O)6]2+
- d)[Fe(H2O)6]2+
Correct answer is option 'A'. Can you explain this answer?
The molar absorptivity at λmax is minimum for:
a)
[Mn (H2O)6]2+
b)
[Cr(H2O)6]2+
c)
[Co(H2O)6]2+
d)
[Fe(H2O)6]2+

|
Isha Bose answered |
A particular wavelength is a measure of how strongly a molecule absorbs light at that wavelength. It is usually denoted by the symbol ε (epsilon) and has units of L mol^-1 cm^-1. The molar absorptivity depends on the molecular structure and the electronic transitions that occur when the molecule absorbs light. It is used in the Beer-Lambert law, which relates the concentration of a solution to its absorbance: A = εcl, where A is the absorbance, c is the concentration, and l is the path length of the solution.
Can you explain the answer of this question below:The complex which exhibits lowest energy electronic absorption band is:- A:[NiCl4]2–
- B:[Ni(H2O)6]2+
- C:[Ni(CN)4]2–
- D:Ni(CO)4
The answer is a.
The complex which exhibits lowest energy electronic absorption band is:
A:
[NiCl4]2–
B:
[Ni(H2O)6]2+
C:
[Ni(CN)4]2–
D:
Ni(CO)4

|
Saikat Ghoshal answered |
The complex which exhibits lowest energy electronic absorption band is [NiCl4]2. This can be explained through the following points:
Explanation:
1. Determining factors for energy of electronic absorption band
The energy of electronic absorption bands depends on various factors such as the nature of the ligand, oxidation state of the metal ion, coordination number, geometry of the complex, and the extent of d-orbital splitting.
2. Comparison of given complexes
Out of the given complexes, [NiCl4]2, [Ni(H2O)6]2, [Ni(CN)4]2, and Ni(CO)4, the complex with the lowest energy of electronic absorption band is [NiCl4]2.
3. Reason for lowest energy in [NiCl4]2
The reason for this can be attributed to the nature of the ligand. Chloride ion being a weak field ligand, it causes less splitting of the d-orbitals of the nickel ion. This results in a lower energy of electronic absorption band as compared to the other complexes where ligands such as water, cyanide, and carbon monoxide cause a greater extent of d-orbital splitting due to their strong field nature.
4. Other factors affecting energy of electronic absorption band
Although the oxidation state of the metal ion, coordination number, and geometry of the complex also affect the energy of electronic absorption bands, in this case, the nature of the ligand is the primary determining factor.
Conclusion:
Thus, [NiCl4]2 is the complex that exhibits the lowest energy of electronic absorption band due to the weak field nature of the chloride ligand causing less splitting of the d-orbitals of the nickel ion.
Explanation:
1. Determining factors for energy of electronic absorption band
The energy of electronic absorption bands depends on various factors such as the nature of the ligand, oxidation state of the metal ion, coordination number, geometry of the complex, and the extent of d-orbital splitting.
2. Comparison of given complexes
Out of the given complexes, [NiCl4]2, [Ni(H2O)6]2, [Ni(CN)4]2, and Ni(CO)4, the complex with the lowest energy of electronic absorption band is [NiCl4]2.
3. Reason for lowest energy in [NiCl4]2
The reason for this can be attributed to the nature of the ligand. Chloride ion being a weak field ligand, it causes less splitting of the d-orbitals of the nickel ion. This results in a lower energy of electronic absorption band as compared to the other complexes where ligands such as water, cyanide, and carbon monoxide cause a greater extent of d-orbital splitting due to their strong field nature.
4. Other factors affecting energy of electronic absorption band
Although the oxidation state of the metal ion, coordination number, and geometry of the complex also affect the energy of electronic absorption bands, in this case, the nature of the ligand is the primary determining factor.
Conclusion:
Thus, [NiCl4]2 is the complex that exhibits the lowest energy of electronic absorption band due to the weak field nature of the chloride ligand causing less splitting of the d-orbitals of the nickel ion.
What is the spin only magnetic moment value in (Bohr Magneton units) of Cr(CO)6?- a)5.92
- b)2.84
- c)4.90
- d)0
Correct answer is option 'D'. Can you explain this answer?
What is the spin only magnetic moment value in (Bohr Magneton units) of Cr(CO)6?
a)
5.92
b)
2.84
c)
4.90
d)
0

|
Yash Roy answered |
The electron configuration is [Ar]3d54s1.We have to accomodate the 6 Ligands and the fact that CO is a strong ligand.
This results in d2sp3 hybridization. Therefore, there are no unpaired electrons in Cr(CO)6. Hence n=0
And the spin only magnetic moment is also 0.
This results in d2sp3 hybridization. Therefore, there are no unpaired electrons in Cr(CO)6. Hence n=0
And the spin only magnetic moment is also 0.
Which of the following pairs of electronic configuration of high–spin transition metal ions (3d) in an octahedral field undergo a substantial Jahn–Teller distortion:- a)d3, d9
- b)d4, d9
- c)d5 , d9
- d)d6 , d9
Correct answer is option 'B'. Can you explain this answer?
Which of the following pairs of electronic configuration of high–spin transition metal ions (3d) in an octahedral field undergo a substantial Jahn–Teller distortion:
a)
d3, d9
b)
d4, d9
c)
d5 , d9
d)
d6 , d9

|
Asf Institute answered |
Jahn-Teller Distortion in Highspin Transition Metal Ions
The Jahn-Teller effect is a phenomenon observed in high-spin transition metal ions with partially filled d-orbitals in an octahedral field. It occurs due to the interaction between electrons and the degenerate molecular orbitals in the octahedral crystal field.
Explanation:
- Highspin transition metal ions have partially filled d-orbitals and exhibit strong electron-electron repulsion.
- When placed in an octahedral field, the d-orbitals split into two sets of degenerate orbitals, known as t2g and eg.
- In highspin ions, the electrons occupy the t2g orbitals before the eg orbitals due to the Hund's rule.
- However, this arrangement is not energetically favorable as the t2g orbitals are more stable than the eg orbitals.
- The Jahn-Teller effect occurs when the degeneracy of the eg orbitals is lifted by a distortion of the octahedral field, leading to a lowering of the energy of the system.
- The distortion can be either elongation or compression of the octahedral field along one of the axes.
- The distortion causes the electrons to occupy the lower energy eg orbital, resulting in a non-degenerate ground state.
- Only those pairs of electronic configurations of highspin transition metal ions that exhibit the Jahn-Teller effect undergo substantial distortion.
- Option 'B' is correct as it represents the pair of electronic configurations d4 and d9, which exhibit the Jahn-Teller effect. The d4 ion has two electrons in the eg orbitals and two in the t2g orbitals, while the d9 ion has one electron in the eg orbitals and four in the t2g orbitals. Both these ions have partially filled eg orbitals, which are energetically unstable and undergo Jahn-Teller distortion to lower the energy.
The Jahn-Teller effect is a phenomenon observed in high-spin transition metal ions with partially filled d-orbitals in an octahedral field. It occurs due to the interaction between electrons and the degenerate molecular orbitals in the octahedral crystal field.
Explanation:
- Highspin transition metal ions have partially filled d-orbitals and exhibit strong electron-electron repulsion.
- When placed in an octahedral field, the d-orbitals split into two sets of degenerate orbitals, known as t2g and eg.
- In highspin ions, the electrons occupy the t2g orbitals before the eg orbitals due to the Hund's rule.
- However, this arrangement is not energetically favorable as the t2g orbitals are more stable than the eg orbitals.
- The Jahn-Teller effect occurs when the degeneracy of the eg orbitals is lifted by a distortion of the octahedral field, leading to a lowering of the energy of the system.
- The distortion can be either elongation or compression of the octahedral field along one of the axes.
- The distortion causes the electrons to occupy the lower energy eg orbital, resulting in a non-degenerate ground state.
- Only those pairs of electronic configurations of highspin transition metal ions that exhibit the Jahn-Teller effect undergo substantial distortion.
- Option 'B' is correct as it represents the pair of electronic configurations d4 and d9, which exhibit the Jahn-Teller effect. The d4 ion has two electrons in the eg orbitals and two in the t2g orbitals, while the d9 ion has one electron in the eg orbitals and four in the t2g orbitals. Both these ions have partially filled eg orbitals, which are energetically unstable and undergo Jahn-Teller distortion to lower the energy.
A magnetic moment of 1.73 BM will be shown by one among the following- a)[Cu(NH3)4]2+
- b)[Ni(CN)4]2–
- c)TiCl4
- d)[CoCl6]4–
Correct answer is option 'A'. Can you explain this answer?
A magnetic moment of 1.73 BM will be shown by one among the following
a)
[Cu(NH3)4]2+
b)
[Ni(CN)4]2–
c)
TiCl4
d)
[CoCl6]4–
|
|
Ritu Singh answered |
The correct answer is Option A.
Electronic configuration of Cu2+ ion in [Cu(NH3)4]2+.
Cu2+ ion =[Ar]3d94s0.
∴Cu2+ ion has one unpaired electron.
Magnetic moment of [Cu(NH3)4]2+ (μ) =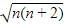 BM
BM
where, n = no. of unpaired electrons
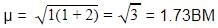
Whereas Ni2+ in [Ni(CN)4]2− , Ti4+ in TiCl4 and Co2+ ion [COCl6]4− has 2,0 and 3 unpaired electrons respectively.
Electronic configuration of Cu2+ ion in [Cu(NH3)4]2+.
Cu2+ ion =[Ar]3d94s0.
∴Cu2+ ion has one unpaired electron.
Magnetic moment of [Cu(NH3)4]2+ (μ) =
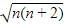 BM
BMwhere, n = no. of unpaired electrons
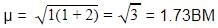
Whereas Ni2+ in [Ni(CN)4]2− , Ti4+ in TiCl4 and Co2+ ion [COCl6]4− has 2,0 and 3 unpaired electrons respectively.
The oxidation number, coordination number and magnetic moment in the following complex is:[Cr(C2O4)2 (NH3)2]-- a)
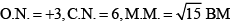
- b)

- c)
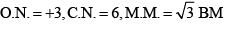
- d)

Correct answer is option 'A'. Can you explain this answer?
The oxidation number, coordination number and magnetic moment in the following complex is:
[Cr(C2O4)2 (NH3)2]-
a)
b)
c)
d)

|
Aryan Gupta answered |
[Cr(C2O4)2 (NH3)2]-
Oxidation number:
x + (-2 x 2) + 0 x 2 = -1
x = +3
Coordination number is 6, because C2O42- bidentate ligand and NH3 monodentate.
Magnetic moment = √n(n+2)
where n is no. of unpaired electrons.
For Cr , n = 3 so Magnetic moment will be √15 BM
Which one the following octahedral complexes will be distorted:- a)[Cr(H2O)6]2+
- b)[Cr(H2O)6]3+
- c)[Mn(H2O)6]2+
- d)[Fe(H2O)6]3+
Correct answer is option 'A'. Can you explain this answer?
Which one the following octahedral complexes will be distorted:
a)
[Cr(H2O)6]2+
b)
[Cr(H2O)6]3+
c)
[Mn(H2O)6]2+
d)
[Fe(H2O)6]3+
|
|
Pooja Choudhury answered |
- [Cr(H2O)6]2+ is distorted because of more number of electrons i.e. 4 electrons (electron-electron repulsion).
- But in the case of Mn & Fe both of them has 3d5 configuration which is more stable & decreases the chances of distortion.
- In the case of Cr3+ less number of electrons are present, so, less electron-electron repulsion hence lesser will be the distortion.
- When there is unsymmetrical filling of electron in Eg orbital occur, the shape will distorted e.g. in the case of d4. This is known as Jahn-Teller distortion.
Hence A is correct.
The correct order of acidity among the following species is:- a)[Na(H2O)6]+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+ > [Sc(H2O)6]3+
- b)[Sc(H2O)6]3+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+ > [Na(H2O)6]+
- c)[Mn(H2O)6]2+ > [Ni(H2O)6]2+ > [Sc(H2O)6]3+ > [Na(H2O)6]+
- d)[Sc(H2O)6]3+ > [Na(H2O)6]+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+
Correct answer is option 'B'. Can you explain this answer?
The correct order of acidity among the following species is:
a)
[Na(H2O)6]+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+ > [Sc(H2O)6]3+
b)
[Sc(H2O)6]3+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+ > [Na(H2O)6]+
c)
[Mn(H2O)6]2+ > [Ni(H2O)6]2+ > [Sc(H2O)6]3+ > [Na(H2O)6]+
d)
[Sc(H2O)6]3+ > [Na(H2O)6]+ > [Ni(H2O)6]2+ > [Mn(H2O)6]2+

|
Edurev.iitjam answered |
Acidity of Aquo Complexes
- Aquo complexes are formed when metal ions are surrounded by water molecules. They are called aquo complexes because water molecules act as ligands in these complexes. The acidity of these aquo complexes depends on the charge on the metal ion and its size.
Order of Acidity
- The order of acidity among the given aquo complexes is determined by the charge on the metal ion. The higher the charge on the metal ion, the more acidic the complex.
- The correct order of acidity among the given species is: [Sc(H2O)6]3 ,[Ni(H2O)6]2 ,[Mn(H2O)6]2 [Na(H2O)6]
Explanation
The correct answer is option B, which places [Sc(H2O)6]3 as the most acidic and [Na(H2O)6] as the least acidic. This is because:
1. [Sc(H2O)6]3 has the highest charge (+3) among the given species, making it the most acidic.
2. [Ni(H2O)6]2 and [Mn(H2O)6]2 have a charge of +2 each, making them less acidic than [Sc(H2O)6]3.
3. [Na(H2O)6] has the lowest charge (+1) among the given species, making it the least acidic.
Hence, the correct order of acidity among the given species is option B.
The correct answer is option B, which places [Sc(H2O)6]3 as the most acidic and [Na(H2O)6] as the least acidic. This is because:
1. [Sc(H2O)6]3 has the highest charge (+3) among the given species, making it the most acidic.
2. [Ni(H2O)6]2 and [Mn(H2O)6]2 have a charge of +2 each, making them less acidic than [Sc(H2O)6]3.
3. [Na(H2O)6] has the lowest charge (+1) among the given species, making it the least acidic.
Hence, the correct order of acidity among the given species is option B.
Which of the following complexes has the highest magnetic moment?
- a)[Fe(CN)6]4-
- b)[VO(H2O)5]2+
- c)[Cr(NH3)6]3+
- d)[Co(NH3)6]3+
Correct answer is option 'C'. Can you explain this answer?
Which of the following complexes has the highest magnetic moment?
a)
[Fe(CN)6]4-
b)
[VO(H2O)5]2+
c)
[Cr(NH3)6]3+
d)
[Co(NH3)6]3+

|
Edurev.iitjam answered |
Higher the number of unpaired electrons, the higher is the magnetic moment. So, the correct option is [Cr(NH3)6]3+
MnCr2O4 is:- a)Normal spinel wit h total CFSE of –15.5 Dq.
- b)Inverse spinel wit h total CFSE of –15.5 Dq.
- c)Normal spinel wit h total CFSE of –24 Dq.
- d)Inverse spinel wit h total CFSE of –24 Dq.
Correct answer is option 'C'. Can you explain this answer?
MnCr2O4 is:
a)
Normal spinel wit h total CFSE of –15.5 Dq.
b)
Inverse spinel wit h total CFSE of –15.5 Dq.
c)
Normal spinel wit h total CFSE of –24 Dq.
d)
Inverse spinel wit h total CFSE of –24 Dq.

|
Edurev.iitjam answered |
In the normal spinel structure, MnCr₂O₄ is arranged as (Mn²⁺) tetrahedral (Cr³⁺)₂ Octahedral. The O²⁻ ions act as weak field ligands.
- For Mn²⁺ (d⁵ configuration), the ion is in a high-spin state. Therefore, its CFSE = 0.
- For Cr³⁺ (d³ configuration) in an octahedral field, the CFSE = -12 Dq per Cr³⁺ ion.
Since there are two Cr³⁺ ions, the total CFSE contributed by Cr³⁺ ions is:
2 × (-12 Dq) = -24 Dq.
Adding these contributions:
CFSE of Mn²⁺ + CFSE of Cr³⁺ = 0 + (-24 Dq) = -24 Dq.
CFSE of Mn²⁺ + CFSE of Cr³⁺ = 0 + (-24 Dq) = -24 Dq.
Thus, MnCr₂O₄ is a normal spinel with a total CFSE of -24 Dq, making Option C correct.
The number of manganese ions in tetrahedral and octahedral sites, respectively in Mn3O4 are:
- a)One Mn2+ and two Mn3+
- b)Two Mn3+ and one Mn2+
- c)One Mn3+ and two Mn2+
- d)Two Mn2+ and one Mn3+
Correct answer is option 'A'. Can you explain this answer?
The number of manganese ions in tetrahedral and octahedral sites, respectively in Mn3O4 are:
a)
One Mn2+ and two Mn3+
b)
Two Mn3+ and one Mn2+
c)
One Mn3+ and two Mn2+
d)
Two Mn2+ and one Mn3+

|
Akshat Saini answered |
Explanation:
Mn3O4 is a mixed oxide of Mn2+ and Mn3+. It has both tetrahedral and octahedral sites. The ratio of Mn2+ and Mn3+ ions in these sites is different.
- Tetrahedral sites: In Mn3O4, there are 8 tetrahedral sites. The Mn2+ ions occupy the tetrahedral sites in a 1:1 ratio with oxygen ions. So, there are 8 Mn2+ ions in the tetrahedral sites.
- Octahedral sites: In Mn3O4, there are 16 octahedral sites. The Mn3+ ions occupy the octahedral sites in a 2:1 ratio with oxygen ions. So, there are 32 Mn3+ ions in the octahedral sites.
Therefore, the number of manganese ions in tetrahedral and octahedral sites, respectively in Mn3O4 are one Mn2+ and two Mn3+. Hence, the correct answer is option A.
Mn3O4 is a mixed oxide of Mn2+ and Mn3+. It has both tetrahedral and octahedral sites. The ratio of Mn2+ and Mn3+ ions in these sites is different.
- Tetrahedral sites: In Mn3O4, there are 8 tetrahedral sites. The Mn2+ ions occupy the tetrahedral sites in a 1:1 ratio with oxygen ions. So, there are 8 Mn2+ ions in the tetrahedral sites.
- Octahedral sites: In Mn3O4, there are 16 octahedral sites. The Mn3+ ions occupy the octahedral sites in a 2:1 ratio with oxygen ions. So, there are 32 Mn3+ ions in the octahedral sites.
Therefore, the number of manganese ions in tetrahedral and octahedral sites, respectively in Mn3O4 are one Mn2+ and two Mn3+. Hence, the correct answer is option A.
The compound which exhibits Jahn-Teller distortion is:
- a)[Co(H2O)6]6+
- b)[Cu(H2O)6]2+
- c)[Mn(H2O)6]2+
- d)[Cr(H2O)6]3+
Correct answer is option 'B'. Can you explain this answer?
The compound which exhibits Jahn-Teller distortion is:
a)
[Co(H2O)6]6+
b)
[Cu(H2O)6]2+
c)
[Mn(H2O)6]2+
d)
[Cr(H2O)6]3+

|
Gaurav Singh answered |
John teller distortion occur only in those compound in which unsymmetrical filling of t2g and eg orbital takes place ..I1st compound had d5 configuration n are symmetrically filled so no jtd.2nd has d4 configuration n high spin complex so its one eg orbital is unsymetrically filled resulting jtd
Option c has d3 configuration and as H20 is weak field ligand so its t2g orbital is symmetrically filled resultin no jtd
Last one has d6 configuration is low spin complex of iron due to strong field nature of pi acceptor ligand cNSo its teg orbital is symmetrically filled reasulting no jtdSo only option 2nd is correct
Option c has d3 configuration and as H20 is weak field ligand so its t2g orbital is symmetrically filled resultin no jtd
Last one has d6 configuration is low spin complex of iron due to strong field nature of pi acceptor ligand cNSo its teg orbital is symmetrically filled reasulting no jtdSo only option 2nd is correct
Cr3+ form for complexes with four different ligands which are [Cr(Cl)6]3–,[Cr(H2O)6]3+, [Cr(NH3)6]3+ and [Cr(CN)6]3–. The order of CFSE (∆0) in these complexes is in the order:- a)
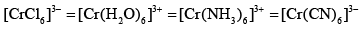
- b)

- c)
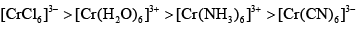
- d)

Correct answer is option 'B'. Can you explain this answer?
Cr3+ form for complexes with four different ligands which are [Cr(Cl)6]3–,[Cr(H2O)6]3+, [Cr(NH3)6]3+ and [Cr(CN)6]3–. The order of CFSE (∆0) in these complexes is in the order:
a)
b)
c)
d)
|
|
Asim Khan answered |
Option b is correct. In all cases cr is in +3 oxidation state. thus d3 system. irrespective of nature of ligand( weak or strong) it should have same cfse
Total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH3)]is- a)3
- b)1
- c)2
- d)4
Correct answer is option 'A'. Can you explain this answer?
Total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH3)]is
a)
3
b)
1
c)
2
d)
4
|
|
Pooja Choudhury answered |
The total number of geometrical isomers for the complex [RhCl(CO)(PPh3)(NH3)] is 3 since it is a square planar.

[Cr(H2O)6]3+ is violet whereas [Cr(NH3)6]3+ is yellow. The wavelength absorbed by [Cr(NH3)6]3+ as compared to that by [Cr(H2O)6]3+ in their absorption spectra will be:- a)Higher
- b)Lower
- c)Same
- d)These complexes will not show any absorption in the visible region
Correct answer is option 'B'. Can you explain this answer?
[Cr(H2O)6]3+ is violet whereas [Cr(NH3)6]3+ is yellow. The wavelength absorbed by [Cr(NH3)6]3+ as compared to that by [Cr(H2O)6]3+ in their absorption spectra will be:
a)
Higher
b)
Lower
c)
Same
d)
These complexes will not show any absorption in the visible region

|
Harshitha Sharma answered |
To calculate the number of moles of CoCl3 in the solution, we need to divide the mass of CoCl3 by its molar mass.
Molar mass of CoCl3 = 129.84 g/mol
Number of moles of CoCl3 = 2.675 g / 129.84 g/mol = 0.0206 mol
Therefore, the solution contains 0.0206 mol of CoCl3.
Molar mass of CoCl3 = 129.84 g/mol
Number of moles of CoCl3 = 2.675 g / 129.84 g/mol = 0.0206 mol
Therefore, the solution contains 0.0206 mol of CoCl3.
It is known that pKa of water is 15.7. Based on this water pKa benchmark, arrange the fo llowing solvated metals–aqua io ns in order of their increasing acidity : Mn2+(H2O)6 , Fe3+(H2O)6, Cu2+(H2O)6 ,Ca2+(H2O)6
- a)All have same acidit ies
- b)Ca2+ < Cu2+ < Mn2+ < Fe3+
- c)Fe3+ < Cu2+ < Mn2+ < Ca2+
- d)Ca2+, Mn2+, Fe3+, Cu2+
Correct answer is option 'D'. Can you explain this answer?
It is known that pKa of water is 15.7. Based on this water pKa benchmark, arrange the fo llowing solvated metals–aqua io ns in order of their increasing acidity : Mn2+(H2O)6 , Fe3+(H2O)6, Cu2+(H2O)6 ,Ca2+(H2O)6
a)
All have same acidit ies
b)
Ca2+ < Cu2+ < Mn2+ < Fe3+
c)
Fe3+ < Cu2+ < Mn2+ < Ca2+
d)
Ca2+, Mn2+, Fe3+, Cu2+

|
Bhavana Dasgupta answered |
I'm sorry, but you haven't provided a list of solvated metals for me to arrange. Could you please provide the list so that I can assist you further?
The ground state term symbols for high spin d5s1 and d5 configurations, respectively are:- a)3S and 6S
- b)6P and 3S
- c)7S and 6S
- d)7P and 6S
Correct answer is option 'C'. Can you explain this answer?
The ground state term symbols for high spin d5s1 and d5 configurations, respectively are:
a)
3S and 6S
b)
6P and 3S
c)
7S and 6S
d)
7P and 6S

|
Tanishq Goyal answered |
Explanation:
For d5s1 configuration, since s=1/2, the total spin can range from 5/2 to 1/2. The total angular momentum can range from 4 to 0.
For d5 configuration, since there are 5 electrons with s=1/2, the total spin can range from 5/2 to 1/2. The total angular momentum can range from 5 to 0.
To determine the ground state term symbol, we need to find the combination of spin and angular momentum that gives the lowest energy.
For d5s1 configuration:
- The lowest spin state is when all electrons are paired, giving a total spin of S=0.
- The lowest angular momentum state is when all electrons occupy the same d orbital, giving a total angular momentum of L=2.
- The ground state term symbol is then 3S, since S=0 and L=2.
For d5 configuration:
- The lowest spin state is when all electrons are paired, giving a total spin of S=0.
- The lowest angular momentum state is when all electrons occupy the same d orbital, giving a total angular momentum of L=5.
- The ground state term symbol is then 6S, since S=0 and L=5.
Therefore, the correct answer is option C, 7S and 6S.
For d5s1 configuration, since s=1/2, the total spin can range from 5/2 to 1/2. The total angular momentum can range from 4 to 0.
For d5 configuration, since there are 5 electrons with s=1/2, the total spin can range from 5/2 to 1/2. The total angular momentum can range from 5 to 0.
To determine the ground state term symbol, we need to find the combination of spin and angular momentum that gives the lowest energy.
For d5s1 configuration:
- The lowest spin state is when all electrons are paired, giving a total spin of S=0.
- The lowest angular momentum state is when all electrons occupy the same d orbital, giving a total angular momentum of L=2.
- The ground state term symbol is then 3S, since S=0 and L=2.
For d5 configuration:
- The lowest spin state is when all electrons are paired, giving a total spin of S=0.
- The lowest angular momentum state is when all electrons occupy the same d orbital, giving a total angular momentum of L=5.
- The ground state term symbol is then 6S, since S=0 and L=5.
Therefore, the correct answer is option C, 7S and 6S.
Among the complexes:(I) K4[Fe(CN)6],
(II) K3[Co(CN)6],
(III) K4[Mn(CN)6], Jahn – Teller distortion is expected in.- a)I, II, and III
- b)III only
- c)I and II
- d)I and III
Correct answer is option 'B'. Can you explain this answer?
Among the complexes:
(I) K4[Fe(CN)6],
(II) K3[Co(CN)6],
(III) K4[Mn(CN)6], Jahn – Teller distortion is expected in.
(II) K3[Co(CN)6],
(III) K4[Mn(CN)6], Jahn – Teller distortion is expected in.
a)
I, II, and III
b)
III only
c)
I and II
d)
I and III

|
Edurev.iitjam answered |
Jahn Teller Distortion in Complexes
- Jahn Teller distortion is a phenomenon that occurs in octahedral complexes with degenerate electronic states. It is caused by the interaction between the electrons in the degenerate orbitals and the molecular vibrations, leading to a distortion in the complex.
Complexes with Jahn Teller Distortion
- Among the given complexes, K4[Fe(CN)6], K3[Co(CN)6], and K4[Mn(CN)6], Jahn Teller distortion is expected only in K4[Mn(CN)6].
- K4[Fe(CN)6] and K3[Co(CN)6] have d6 electronic configuration which is not degenerate and hence do not show Jahn Teller distortion. On the other hand, K4[Mn(CN)6] has d5 electronic configuration which is degenerate. Hence, it shows Jahn Teller distortion.
In conclusion, Jahn Teller distortion is a phenomenon that occurs only in octahedral complexes with degenerate electronic states. Among the given complexes, K4[Mn(CN)6] is the only one with degenerate electronic states and hence shows Jahn Teller distortion.
The number of ligands which have strong crystal field splitting thanH2O among SCN-, NCS-, EDTA4- , 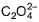 ,
, 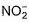 , Br-, PPh3, F-
, Br-, PPh3, F-
Correct answer is '4'. Can you explain this answer?
The number of ligands which have strong crystal field splitting than
H2O among SCN-, NCS-, EDTA4- , 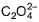 ,
, 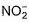 , Br-, PPh3, F-
, Br-, PPh3, F-

|
Learners Habitat answered |
NCS- edta4- , 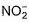 and PPh3 are strong field ligand than H2O.
and PPh3 are strong field ligand than H2O.
The correct statement about the Cu–N bond distance in [Cu(NH3)6]2+ is:- a)All the bond distance are equal.
- b)The axial bonds are longer than the equatorial ones.
- c)The equatorial bonds are longer than the axial ones.
- d)All the bond distances are unequal.
Correct answer is option 'B'. Can you explain this answer?
The correct statement about the Cu–N bond distance in [Cu(NH3)6]2+ is:
a)
All the bond distance are equal.
b)
The axial bonds are longer than the equatorial ones.
c)
The equatorial bonds are longer than the axial ones.
d)
All the bond distances are unequal.

|
Edurev.iitjam answered |
- In [Cu(NH3)6]2+, there are six NH3 ligands arranged in an octahedral geometry around the central Cu2+ ion.
- The bond distances are different due to the difference in the orientation of the ligands.
- There are two types of bond distances in this complex: axial and equatorial.
- Axial bonds: These are the bonds that are perpendicular to the plane of the equatorial bonds. There are two axial bonds in this complex.
- Equatorial bonds: These are the bonds that lie in the plane of the complex. There are four equatorial bonds in this complex.
- The repulsion between the lone pairs of electrons on the NH3 ligands causes the axial bonds to be longer than the equatorial bonds.
- Therefore, the correct statement is that the axial bonds are longer than the equatorial ones (option B is correct).
- The bond distances are different due to the difference in the orientation of the ligands.
- There are two types of bond distances in this complex: axial and equatorial.
- Axial bonds: These are the bonds that are perpendicular to the plane of the equatorial bonds. There are two axial bonds in this complex.
- Equatorial bonds: These are the bonds that lie in the plane of the complex. There are four equatorial bonds in this complex.
- The repulsion between the lone pairs of electrons on the NH3 ligands causes the axial bonds to be longer than the equatorial bonds.
- Therefore, the correct statement is that the axial bonds are longer than the equatorial ones (option B is correct).
The enthalpies of hydration of Ca2+, Mn2+, Zn2+ follow the order:- a)Mn2+ > Ca2+ > Zn2+
- b)Zn2+ > Ca2+ > Mn2+
- c)Mn2+ > Zn2+ > Ca2+
- d)Zn2+ > Mn2+ > Ca2+
Correct answer is option 'D'. Can you explain this answer?
The enthalpies of hydration of Ca2+, Mn2+, Zn2+ follow the order:
a)
Mn2+ > Ca2+ > Zn2+
b)
Zn2+ > Ca2+ > Mn2+
c)
Mn2+ > Zn2+ > Ca2+
d)
Zn2+ > Mn2+ > Ca2+

|
Asf Institute answered |
Enthalpy of Hydration
- Enthalpy of hydration is the amount of energy released when one mole of an ion in the gaseous state dissolves in water to form an aqueous solution.
Order of Enthalpies of Hydration
- The order of enthalpies of hydration of Ca2+, Mn2+, Zn2+ can be determined by considering the size and charge of the ions.
- Ca2+ has a larger size than Mn2+ and Zn2+, so it has a weaker hydration energy than the other two.
- Mn2+ has a smaller size than Zn2+, but it has a greater charge density due to its higher charge. This results in a stronger hydration energy for Mn2+ than Zn2+.
- Zn2+ has the smallest size and charge, resulting in the strongest hydration energy.
- Therefore, the order of the enthalpies of hydration is Zn2+ > Mn2+ > Ca2+.
The correct answer is option D, which lists the order of enthalpies of hydration as Zn2+, Mn2+, Ca2+.
According to the crystal field theory, Ni2+ can have two unpaired electron in:- a)Octahedral geo metry only
- b)Tetrahedral geometry only
- c)Square planar geometry only
- d)Both tetrahedral and octahedral geometry
Correct answer is option 'D'. Can you explain this answer?
According to the crystal field theory, Ni2+ can have two unpaired electron in:
a)
Octahedral geo metry only
b)
Tetrahedral geometry only
c)
Square planar geometry only
d)
Both tetrahedral and octahedral geometry

|
Edurev.iitjam answered |
Crystal Field Theory and Unpaired Electrons
- The crystal field theory is a model used to explain the behavior of transition metal ions in coordination compounds. It is based on the idea that the ligands surrounding the central metal ion create a crystal field that affects the energy levels of the metal ion's d orbitals. In particular, the crystal field splits the d orbitals into two sets of energy levels, with some orbitals raised in energy and others lowered.
- One consequence of this splitting is that some of the d orbitals may become partially filled, leading to unpaired electrons. These unpaired electrons can have important consequences for the properties of the compound, such as its magnetic behavior.
Ni2+ and Unpaired Electrons
- Ni2+ is a transition metal ion with a d8 electron configuration. This means it has eight electrons in its d orbitals, and in the absence of ligands, these electrons would pair up in the lower energy levels.
- However, when Ni2+ is coordinated to ligands, the crystal field splits the d orbitals into two sets of energy levels. In particular, the t2g set of orbitals is lowered in energy, while the eg set is raised. This means that if Ni2+ is in a geometry where the ligands are close enough to cause significant splitting, some of the d orbitals may be partially filled, leading to unpaired electrons.
Tetrahedral and Octahedral Geometries
- The crystal field splitting is affected by the geometry of the ligands around the central metal ion. In particular, the splitting is greater for octahedral geometries than for tetrahedral geometries. This means that for Ni2+, the eg set of orbitals is more likely to be raised above the t2g set in an octahedral geometry, leading to unpaired electrons.
- However, even in a tetrahedral geometry, there can still be some splitting of the d orbitals, leading to the possibility of unpaired electrons. This means that Ni2+ can have two unpaired electrons in both tetrahedral and octahedral geometries.
Conclusion
- In summary, the crystal field theory can be used to predict whether a transition metal ion will have unpaired electrons in a coordination compound. For Ni2+, the geometry of the ligands around the central metal ion affects the magnitude of the crystal field splitting, but even in a tetrahedral geometry, there can still be some splitting, leading to unpaired electrons. Therefore, the correct answer is option D, both tetrahedral and octahedral geometries.
The pair of compounds having metals in their highest oxidation state is
- a)MnO2, FeCl3
- b)[MnO4]–, CrO2Cl2
- c)[Fe(CN)6]3–, [Co(CN)3]
- d)[NiCl4]2–, [CoCl4]–
Correct answer is option 'B'. Can you explain this answer?
The pair of compounds having metals in their highest oxidation state is
a)
MnO2, FeCl3
b)
[MnO4]–, CrO2Cl2
c)
[Fe(CN)6]3–, [Co(CN)3]
d)
[NiCl4]2–, [CoCl4]–

|
Akanksha Choudhary answered |
-, [FeCl6]3-
c)KMnO4, Fe2O3
d)MnSO4, FeCO3
Answer: b) [MnO4]-, [FeCl6]3-
c)KMnO4, Fe2O3
d)MnSO4, FeCO3
Answer: b) [MnO4]-, [FeCl6]3-
The square planar geometry is based on- a)Z-exclusion principle
- b)Electroneutrality principle
- c)None of the above
- d)Pauli exclusion principle
Correct answer is option 'A'. Can you explain this answer?
The square planar geometry is based on
a)
Z-exclusion principle
b)
Electroneutrality principle
c)
None of the above
d)
Pauli exclusion principle

|
Soumya Sengupta answered |
Introduction:
The square planar geometry is a type of molecular geometry that describes the arrangement of atoms in a molecule. In this geometry, the central atom is surrounded by four ligands that are arranged in a square plane around it. The square planar geometry is commonly observed in transition metal complexes and some organic molecules.
Explanation:
The correct answer to the question is option 'A', which states that the square planar geometry is based on the Z-exclusion principle. Let's understand why this is the correct answer.
Z-exclusion principle:
The Z-exclusion principle, also known as the 18-electron rule, is a concept in chemistry that describes the stability of transition metal complexes based on the number of valence electrons present. According to this principle, stable transition metal complexes tend to have 18 valence electrons surrounding the central metal atom.
Electronic configuration:
In the square planar geometry, the central atom is typically a transition metal, which has d orbitals available for bonding. The electronic configuration of transition metals allows them to form coordination complexes by donating and accepting electron pairs.
Ligands in square planar geometry:
In a square planar geometry, the central atom is surrounded by four ligands arranged in a square plane. These ligands can be either negatively charged (anions) or neutral molecules. The ligands form coordinate covalent bonds with the central atom by donating a pair of electrons.
Electronegativity of ligands:
The Z-exclusion principle is based on the fact that the ligands in a transition metal complex are negatively charged or neutral. This is because ligands with positive charges would be highly electronegative and would not form stable bonds with the metal atom. Hence, the Z-exclusion principle ensures that the ligands are electroneutral or negatively charged to maintain stability.
Conclusion:
In conclusion, the square planar geometry is based on the Z-exclusion principle, which describes the stability of transition metal complexes based on the number of valence electrons present. This principle ensures that the ligands surrounding the central metal atom in a square planar geometry are electroneutral or negatively charged, maintaining stability in the complex.
The square planar geometry is a type of molecular geometry that describes the arrangement of atoms in a molecule. In this geometry, the central atom is surrounded by four ligands that are arranged in a square plane around it. The square planar geometry is commonly observed in transition metal complexes and some organic molecules.
Explanation:
The correct answer to the question is option 'A', which states that the square planar geometry is based on the Z-exclusion principle. Let's understand why this is the correct answer.
Z-exclusion principle:
The Z-exclusion principle, also known as the 18-electron rule, is a concept in chemistry that describes the stability of transition metal complexes based on the number of valence electrons present. According to this principle, stable transition metal complexes tend to have 18 valence electrons surrounding the central metal atom.
Electronic configuration:
In the square planar geometry, the central atom is typically a transition metal, which has d orbitals available for bonding. The electronic configuration of transition metals allows them to form coordination complexes by donating and accepting electron pairs.
Ligands in square planar geometry:
In a square planar geometry, the central atom is surrounded by four ligands arranged in a square plane. These ligands can be either negatively charged (anions) or neutral molecules. The ligands form coordinate covalent bonds with the central atom by donating a pair of electrons.
Electronegativity of ligands:
The Z-exclusion principle is based on the fact that the ligands in a transition metal complex are negatively charged or neutral. This is because ligands with positive charges would be highly electronegative and would not form stable bonds with the metal atom. Hence, the Z-exclusion principle ensures that the ligands are electroneutral or negatively charged to maintain stability.
Conclusion:
In conclusion, the square planar geometry is based on the Z-exclusion principle, which describes the stability of transition metal complexes based on the number of valence electrons present. This principle ensures that the ligands surrounding the central metal atom in a square planar geometry are electroneutral or negatively charged, maintaining stability in the complex.
What is the oxidation state of platinum in [PtCl6]2-- a)+ 2
- b)+ 4
- c)+ 6
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
What is the oxidation state of platinum in [PtCl6]2-
a)
+ 2
b)
+ 4
c)
+ 6
d)
None of the above
|
|
Vivek Khatri answered |
x + 6 X – 1 = – 2
x – 6 = – 2
x = 6 – 2
x = + 4
Hence, the oxidation state of platinum in [PtCl6]2- is + 4.
x – 6 = – 2
x = 6 – 2
x = + 4
Hence, the oxidation state of platinum in [PtCl6]2- is + 4.
The zero magnetic moment of octahedral K2NiF6 is due to- a)Low spin d6Ni(IV) complex
- b)Low spin d8Ni(IV) complex
- c)High spin d8Ni(IV) complex
- d)High spin d6Ni(IV) complex
Correct answer is option 'A'. Can you explain this answer?
The zero magnetic moment of octahedral K2NiF6 is due to
a)
Low spin d6Ni(IV) complex
b)
Low spin d8Ni(IV) complex
c)
High spin d8Ni(IV) complex
d)
High spin d6Ni(IV) complex

|
Pioneer Academy answered |
A low spin complex is formed when the ligands are of strong field strength whereas high spin is characterised by weak field strength.
In K2NiF6 , Ni is in +4 oxidation state with d6 electronic configuration.Here fluoride acts as a strong field ligand resulting in low spin complex.
In a strong field, all of the electrons in d6 are paired up, leaving zero unpaired electrons, corresponding to a zero magnetic moment.
Note: When metal is in +4 oxidation state all ligands act as strong field ligands.
In K2NiF6 , Ni is in +4 oxidation state with d6 electronic configuration.Here fluoride acts as a strong field ligand resulting in low spin complex.
In a strong field, all of the electrons in d6 are paired up, leaving zero unpaired electrons, corresponding to a zero magnetic moment.
Note: When metal is in +4 oxidation state all ligands act as strong field ligands.
When EDTA solution is added to Mg2+ ion solution, then which of the following statements is not true?- a)Colorless [Mg–EDTA]2– chelate is formed
- b)All six coordinate sites of Mg2+ are occupied
- c)pH of the solution is decreased
- d)four coordinate sites of Mg2+ are occupied by EDTA and remaining two sites are occupied by water molecule
Correct answer is option 'D'. Can you explain this answer?
When EDTA solution is added to Mg2+ ion solution, then which of the following statements is not true?
a)
Colorless [Mg–EDTA]2– chelate is formed
b)
All six coordinate sites of Mg2+ are occupied
c)
pH of the solution is decreased
d)
four coordinate sites of Mg2+ are occupied by EDTA and remaining two sites are occupied by water molecule
|
|
Pooja Choudhury answered |
- When EDTA solution is added to the Mg2+ ion solution, all six-coordinate sites of Mg2+ are occupied, and a colourless [Mg−EDTA]2− chelate is formed. This reaction also results in the lowering of the pH of the solution.
- So, the correct answer is 'Four coordinates sites of Mg2+ are occupied by EDTA and the remaining two sites are occupied by water molecules.'
Which of the following complex is high spin?- a)K4[Fe(CN)6]
- b)[PtCl4]2–
- c)[CoF6]3–
- d)[Ni(NH3]6]2+
Correct answer is option 'C'. Can you explain this answer?
Which of the following complex is high spin?
a)
K4[Fe(CN)6]
b)
[PtCl4]2–
c)
[CoF6]3–
d)
[Ni(NH3]6]2+

|
Anushka Basak answered |
A) K4[Fe(CN)6] is a low spin complex because Fe(II) has a d6 configuration with electrons pairing up in the lower energy d orbitals before pairing in the higher energy orbitals.
b) [PtCl4]2- is a high spin complex because Pt(II) has a d8 configuration with unpaired electrons that occupy the higher energy orbitals.
b) [PtCl4]2- is a high spin complex because Pt(II) has a d8 configuration with unpaired electrons that occupy the higher energy orbitals.
Select correct statement (s) regarding octahedron complex having CFSE = –1.2∆0.- a)Compound is neither low spin nor high spin complex.
- b)Type of hybridization complex does not depend upon nature of ligands.
- c)Magnetic moment of complex compounds is either
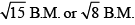
- d)All are incorrect statements.
Correct answer is option 'A,B,C'. Can you explain this answer?
Select correct statement (s) regarding octahedron complex having CFSE = –1.2∆0.
a)
Compound is neither low spin nor high spin complex.
b)
Type of hybridization complex does not depend upon nature of ligands.
c)
Magnetic moment of complex compounds is either 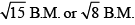
d)
All are incorrect statements.

|
Mayank Shekhawat answered |
D3 and d8 will give this cfse value
both does not have low or high spin.
same for strong and weak ligand.
and have 2 or 3 unpaired electron
both does not have low or high spin.
same for strong and weak ligand.
and have 2 or 3 unpaired electron
CFSE of transition metal complexes can be determined by:- a)UV visible spectroscopy
- b)Microwave spectroscopy
- c)IR spectroscopy
- d)NMR spectroscopy
Correct answer is option 'A'. Can you explain this answer?
CFSE of transition metal complexes can be determined by:
a)
UV visible spectroscopy
b)
Microwave spectroscopy
c)
IR spectroscopy
d)
NMR spectroscopy

|
Sinjini Nair answered |
UV Visible Spectroscopy as a Method to Determine CFSE of Transition Metal Complexes
UV visible spectroscopy is a powerful technique used to determine the crystal field splitting energy (CFSE) of transition metal complexes. This method involves the use of light to study the electronic transitions in the metal-ligand bond, which provides valuable information about the CFSE and the electronic structure of the complex.
The principle of UV-visible spectroscopy is based on the fact that when light is absorbed by a molecule, it promotes an electron from a lower energy level to a higher energy level, resulting in the formation of an excited state. The energy difference between the ground state and the excited state is related to the CFSE of the complex.
Steps Involved in Determining CFSE of Transition Metal Complexes using UV-Visible Spectroscopy:
1. Preparation of Samples: The first step in determining CFSE of a transition metal complex using UV-Visible spectroscopy is the preparation of the sample. The sample must be carefully prepared to ensure that it is homogeneous and free from any impurities.
2. Measurement of Absorbance: Once the sample is prepared, the UV-Visible spectrum of the complex is obtained. The spectrum is obtained by measuring the absorbance of the sample at different wavelengths of light. The absorbance is then plotted against the wavelength to produce a spectrum.
3. Analysis of Spectrum: The spectrum is then analyzed to determine the CFSE of the complex. The CFSE is determined from the energy difference between the ground state and the excited state. The lower the energy difference, the higher the CFSE.
4. Comparison with Theoretical Values: The value obtained for the CFSE is then compared with the theoretical value to determine the accuracy of the measurement.
Advantages of Using UV-Visible Spectroscopy to Determine CFSE:
1. Non-destructive: UV-Visible spectroscopy is a non-destructive method, which means that the sample can be used for further analysis.
2. High sensitivity: UV-Visible spectroscopy is a highly sensitive technique, which means that it can detect even small changes in the electronic structure of the complex.
3. Easy to use: UV-Visible spectroscopy is a relatively simple and easy-to-use technique, which makes it accessible to a wide range of researchers.
Conclusion:
In conclusion, UV-Visible spectroscopy is a powerful technique that can be used to determine the CFSE of transition metal complexes. This method provides valuable information about the electronic structure of the complex, which can be used to design new materials with specific properties.
UV visible spectroscopy is a powerful technique used to determine the crystal field splitting energy (CFSE) of transition metal complexes. This method involves the use of light to study the electronic transitions in the metal-ligand bond, which provides valuable information about the CFSE and the electronic structure of the complex.
The principle of UV-visible spectroscopy is based on the fact that when light is absorbed by a molecule, it promotes an electron from a lower energy level to a higher energy level, resulting in the formation of an excited state. The energy difference between the ground state and the excited state is related to the CFSE of the complex.
Steps Involved in Determining CFSE of Transition Metal Complexes using UV-Visible Spectroscopy:
1. Preparation of Samples: The first step in determining CFSE of a transition metal complex using UV-Visible spectroscopy is the preparation of the sample. The sample must be carefully prepared to ensure that it is homogeneous and free from any impurities.
2. Measurement of Absorbance: Once the sample is prepared, the UV-Visible spectrum of the complex is obtained. The spectrum is obtained by measuring the absorbance of the sample at different wavelengths of light. The absorbance is then plotted against the wavelength to produce a spectrum.
3. Analysis of Spectrum: The spectrum is then analyzed to determine the CFSE of the complex. The CFSE is determined from the energy difference between the ground state and the excited state. The lower the energy difference, the higher the CFSE.
4. Comparison with Theoretical Values: The value obtained for the CFSE is then compared with the theoretical value to determine the accuracy of the measurement.
Advantages of Using UV-Visible Spectroscopy to Determine CFSE:
1. Non-destructive: UV-Visible spectroscopy is a non-destructive method, which means that the sample can be used for further analysis.
2. High sensitivity: UV-Visible spectroscopy is a highly sensitive technique, which means that it can detect even small changes in the electronic structure of the complex.
3. Easy to use: UV-Visible spectroscopy is a relatively simple and easy-to-use technique, which makes it accessible to a wide range of researchers.
Conclusion:
In conclusion, UV-Visible spectroscopy is a powerful technique that can be used to determine the CFSE of transition metal complexes. This method provides valuable information about the electronic structure of the complex, which can be used to design new materials with specific properties.
Chapter doubts & questions for Coordination Chemistry (d block) - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Coordination Chemistry (d block) - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily