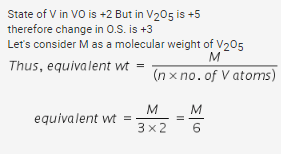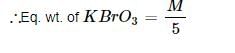All Exams >
Chemistry >
Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry >
All Questions
All questions of Mole Concept, Volumetric & Redox for Chemistry Exam
4.4g of CO2 and 2.24 litre of H2 at STP(273.15 K and 1 atm pressure)are mixed in a container.The total of molecules present in the container will be
- a)6.023 × 1023
- b)12.044 × 1022
- c)6.023 × 1022
- d)6.023 ×1024
Correct answer is 'B'. Can you explain this answer?
4.4g of CO2 and 2.24 litre of H2 at STP(273.15 K and 1 atm pressure)are mixed in a container.The total of molecules present in the container will be
a)
6.023 × 1023
b)
12.044 × 1022
c)
6.023 × 1022
d)
6.023 ×1024
|
|
Anjana Sharma answered |
Moles of CO2=4.4/44=0.1, moles of H2=2.24/22.4=0.1 ; therefore total moles of gases =0.2 and molecules are 0.2 x Na = 0.2 x 6 x10^23=1.2 x 10^23.
How many gm of I2 are present in a solution which requires 40 ml of 0.11N Na2S2O3 to react with it via the reaction : S2O32– + I2 → S4O62– + 2I– ?- a)12.7g
- b)0.558g
- c)25.4g
- d)11.4g
Correct answer is option 'B'. Can you explain this answer?
How many gm of I2 are present in a solution which requires 40 ml of 0.11N Na2S2O3 to react with it via the reaction : S2O32– + I2 → S4O62– + 2I– ?
a)
12.7g
b)
0.558g
c)
25.4g
d)
11.4g
|
|
Vikram Kapoor answered |
Meq. of I2 = Meq. of Na2S2O3 = 40 × 0.11
∴ (W × 2 × 1000)/254 = 40 × 0.11
W of I2 = 0.558g
∴ (W × 2 × 1000)/254 = 40 × 0.11
W of I2 = 0.558g
4I– + Hg2+ → HgI42– ; 1 mole of each Hg2+ and I– will form:- a)1 mole of HgI42–
- b)0.5 mole of HgI42–
- c)0.25 mole of HgI42–
- d)2 mole of HgI42–
Correct answer is option 'C'. Can you explain this answer?
4I– + Hg2+ → HgI42– ; 1 mole of each Hg2+ and I– will form:
a)
1 mole of HgI42–
b)
0.5 mole of HgI42–
c)
0.25 mole of HgI42–
d)
2 mole of HgI42–
|
|
Rajeev Sharma answered |
Balanced reaction is as follows :
Hg2+(aq) + 4I- (aq) ⇋ HgI4 2- (aq)
Thus,
4 mole of I- produces = 1 mole of HgI4 (2-)
1 mole I- produces = 1/4 moles = 0.25 mole of HgI4 (2-)
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g of elemental boron (at. mass = 10.8) from the reduction of boron trichloride with hydrogen:- a)44.8 L
- b)22.4 L
- c)89.6 L
- d)67.2 L
Correct answer is option 'D'. Can you explain this answer?
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g of elemental boron (at. mass = 10.8) from the reduction of boron trichloride with hydrogen:
a)
44.8 L
b)
22.4 L
c)
89.6 L
d)
67.2 L

|
Stuti Patel answered |
Calculation of Volume of Hydrogen Gas Consumed in Obtaining Elemental Boron
Given:
Amount of boron (B) obtained = 21.6 g
Atomic mass of boron (B) = 10.8
Pressure (P) = 1 atm
Temperature (T) = 273 K
We can use the balanced chemical equation for the reduction of boron trichloride (BCl3) with hydrogen (H2) to obtain elemental boron (B):
BCl3 + 3H2 → B + 3HCl
From the equation, we can see that one mole of boron is obtained by reacting 3 moles of hydrogen gas. Therefore, we can use the following steps to calculate the volume of hydrogen gas consumed:
Step 1: Calculate the number of moles of boron obtained
Number of moles of B = Mass of B / Atomic mass of B
Number of moles of B = 21.6 g / 10.8 g/mol
Number of moles of B = 2 mol
Step 2: Calculate the number of moles of hydrogen gas required
From the balanced equation, we know that 3 moles of H2 react with 1 mole of B. Therefore, the number of moles of H2 required is:
Number of moles of H2 = 3 x Number of moles of B
Number of moles of H2 = 3 x 2 mol
Number of moles of H2 = 6 mol
Step 3: Calculate the volume of hydrogen gas consumed
Using the ideal gas law, we can calculate the volume of hydrogen gas consumed:
PV = nRT
where:
P = Pressure = 1 atm
V = Volume of H2
n = Number of moles of H2 = 6 mol
R = Gas constant = 0.0821 L atm mol^-1 K^-1
T = Temperature = 273 K
Rearranging the equation, we get:
V = nRT / P
V = 6 mol x 0.0821 L atm mol^-1 K^-1 x 273 K / 1 atm
V = 134.52 L
Therefore, the volume of hydrogen gas consumed in obtaining 21.6 g of elemental boron is 134.52 L. However, the question asks for the volume of hydrogen gas at STP (standard temperature and pressure), which is 0°C (273 K) and 1 atm. At STP, one mole of any ideal gas occupies 22.4 L. Therefore, we need to convert the volume of hydrogen gas at the given conditions to STP:
Volume of H2 at STP = (134.52 L / 6 mol) x (1 mol / 22.4 L)
Volume of H2 at STP = 1.20 L/mol x 6 mol
Volume of H2 at STP = 7.20 L
Therefore, the volume of hydrogen gas at STP consumed in obtaining 21.6 g of elemental boron is 7.20 L, which is option (d) in the given choices.
Given:
Amount of boron (B) obtained = 21.6 g
Atomic mass of boron (B) = 10.8
Pressure (P) = 1 atm
Temperature (T) = 273 K
We can use the balanced chemical equation for the reduction of boron trichloride (BCl3) with hydrogen (H2) to obtain elemental boron (B):
BCl3 + 3H2 → B + 3HCl
From the equation, we can see that one mole of boron is obtained by reacting 3 moles of hydrogen gas. Therefore, we can use the following steps to calculate the volume of hydrogen gas consumed:
Step 1: Calculate the number of moles of boron obtained
Number of moles of B = Mass of B / Atomic mass of B
Number of moles of B = 21.6 g / 10.8 g/mol
Number of moles of B = 2 mol
Step 2: Calculate the number of moles of hydrogen gas required
From the balanced equation, we know that 3 moles of H2 react with 1 mole of B. Therefore, the number of moles of H2 required is:
Number of moles of H2 = 3 x Number of moles of B
Number of moles of H2 = 3 x 2 mol
Number of moles of H2 = 6 mol
Step 3: Calculate the volume of hydrogen gas consumed
Using the ideal gas law, we can calculate the volume of hydrogen gas consumed:
PV = nRT
where:
P = Pressure = 1 atm
V = Volume of H2
n = Number of moles of H2 = 6 mol
R = Gas constant = 0.0821 L atm mol^-1 K^-1
T = Temperature = 273 K
Rearranging the equation, we get:
V = nRT / P
V = 6 mol x 0.0821 L atm mol^-1 K^-1 x 273 K / 1 atm
V = 134.52 L
Therefore, the volume of hydrogen gas consumed in obtaining 21.6 g of elemental boron is 134.52 L. However, the question asks for the volume of hydrogen gas at STP (standard temperature and pressure), which is 0°C (273 K) and 1 atm. At STP, one mole of any ideal gas occupies 22.4 L. Therefore, we need to convert the volume of hydrogen gas at the given conditions to STP:
Volume of H2 at STP = (134.52 L / 6 mol) x (1 mol / 22.4 L)
Volume of H2 at STP = 1.20 L/mol x 6 mol
Volume of H2 at STP = 7.20 L
Therefore, the volume of hydrogen gas at STP consumed in obtaining 21.6 g of elemental boron is 7.20 L, which is option (d) in the given choices.
20 ml of xM HCl neutralizes completely 10 ml of 0.1M NaHCO3 and a further 5ml of 0.2M Na2CO3 solution to methyl orange end point. The value of x is:- a)0.167M
- b)0.133M
- c)0.150M
- d)0.200M
Correct answer is option 'C'. Can you explain this answer?
20 ml of xM HCl neutralizes completely 10 ml of 0.1M NaHCO3 and a further 5ml of 0.2M Na2CO3 solution to methyl orange end point. The value of x is:
a)
0.167M
b)
0.133M
c)
0.150M
d)
0.200M
|
|
Neha Choudhury answered |
apply concept of dilution of solution..MV=mv
"M" & "V" are molality and volume before dilution and "m" & "v" after dilution respectively
Correct option C
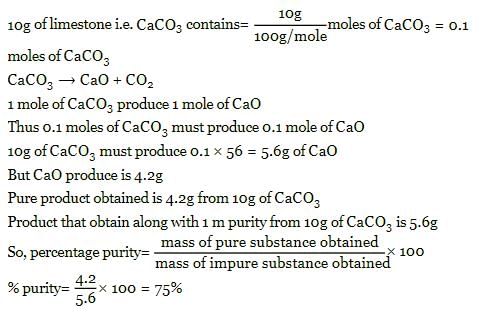
33.6g of an impure sample of sodium bicarbonate when heated strongly gave 4.4g of CO2. The % purity of NaHCO3 would be:- a)25%
- b)50%
- c)75%
- d)100%
Correct answer is option 'B'. Can you explain this answer?
33.6g of an impure sample of sodium bicarbonate when heated strongly gave 4.4g of CO2. The % purity of NaHCO3 would be:
a)
25%
b)
50%
c)
75%
d)
100%

|
Madhavan Iyer answered |
2NaHCO3 = Na2CO3 + CO2 + H2O
Moles of CO2 = 4.4/44 =0.1
Then moles of NaHCO3 =0.1×2/1 =0.2
And wt. Of NaHCO3 = 0.2 × 84 = 16.8gm
%purity of NaHCO3 = 16.8 × 100/33.6 =50%.
Moles of CO2 = 4.4/44 =0.1
Then moles of NaHCO3 =0.1×2/1 =0.2
And wt. Of NaHCO3 = 0.2 × 84 = 16.8gm
%purity of NaHCO3 = 16.8 × 100/33.6 =50%.
0.16g of dibasic acid required 25ml of N/10 NaOH for complete neutralization. Molecular wt. of acid is:- a)32
- b)64
- c)128
- d)256
Correct answer is option 'C'. Can you explain this answer?
0.16g of dibasic acid required 25ml of N/10 NaOH for complete neutralization. Molecular wt. of acid is:
a)
32
b)
64
c)
128
d)
256

|
Anirban Khanna answered |
At neutralisation,
25 ml N/10 NaOH ≡≡25 ml N/10 acid
Now,
25 ml N/10 acid = 0.16 gm
So,
1000 ml N/10 acid = (0.16/25) x 1000
= 6.4 g
Therefore,
1000 ml N acid = 6.4 x 10 = 64 g
Thus, the equivalent weight of acid = 64 g
Since the acid is dibasic,
the molecular weight of acid = 64 x 2 = 128 g
A molal solution is one that contains one mole of solute in- a)1000 g of solvent
- b)1.0 L of solvent
- c)1.0 L of solution
- d)22.4 L of solution
Correct answer is option 'A'. Can you explain this answer?
A molal solution is one that contains one mole of solute in
a)
1000 g of solvent
b)
1.0 L of solvent
c)
1.0 L of solution
d)
22.4 L of solution

|
Pranavi Mishra answered |
Explanation:
A molal solution is a solution that contains one mole of solute dissolved in 1000 grams (1 kilogram) of solvent. The term "molal" refers to the concentration of the solute in the solvent. It is different from molar concentration, which is the amount of solute dissolved in a given volume of solution.
Understanding Molality:
Molality (m) is defined as the number of moles of solute per kilogram of solvent. It is expressed in units of moles per kilogram (mol/kg). Molality is a useful way to express concentration, especially in situations where temperature changes can affect the volume of the solution.
Why Option 'A' is Correct:
Option 'A' states that a molal solution contains one mole of solute in 1000 grams of solvent. This is the correct definition of a molal solution. By definition, a molal solution has a molality of 1 mol/kg, which means that there is one mole of solute dissolved in one kilogram of solvent.
Explanation of Other Options:
- Option 'B' states that a molal solution contains one mole of solute in 1.0 liter of solvent. This is incorrect because a liter is a measure of volume, not mass. Molality is based on the mass of the solvent, not its volume.
- Option 'C' states that a molal solution contains one mole of solute in 1.0 liter of solution. This is incorrect because molality is a measure of the concentration of the solute in the solvent only, not in the entire solution.
- Option 'D' states that a molal solution contains one mole of solute in 22.4 liters of solution. This is incorrect because the volume of the solution is not relevant to molality. Molality is based on the mass of the solvent, not the volume of the solution.
Therefore, the correct answer is option 'A' - a molal solution contains one mole of solute in 1000 grams (1 kilogram) of solvent.
A molal solution is a solution that contains one mole of solute dissolved in 1000 grams (1 kilogram) of solvent. The term "molal" refers to the concentration of the solute in the solvent. It is different from molar concentration, which is the amount of solute dissolved in a given volume of solution.
Understanding Molality:
Molality (m) is defined as the number of moles of solute per kilogram of solvent. It is expressed in units of moles per kilogram (mol/kg). Molality is a useful way to express concentration, especially in situations where temperature changes can affect the volume of the solution.
Why Option 'A' is Correct:
Option 'A' states that a molal solution contains one mole of solute in 1000 grams of solvent. This is the correct definition of a molal solution. By definition, a molal solution has a molality of 1 mol/kg, which means that there is one mole of solute dissolved in one kilogram of solvent.
Explanation of Other Options:
- Option 'B' states that a molal solution contains one mole of solute in 1.0 liter of solvent. This is incorrect because a liter is a measure of volume, not mass. Molality is based on the mass of the solvent, not its volume.
- Option 'C' states that a molal solution contains one mole of solute in 1.0 liter of solution. This is incorrect because molality is a measure of the concentration of the solute in the solvent only, not in the entire solution.
- Option 'D' states that a molal solution contains one mole of solute in 22.4 liters of solution. This is incorrect because the volume of the solution is not relevant to molality. Molality is based on the mass of the solvent, not the volume of the solution.
Therefore, the correct answer is option 'A' - a molal solution contains one mole of solute in 1000 grams (1 kilogram) of solvent.
In a compound C, H, N atoms are present in 9 : 1 : 3.5 by weight. Molecular weight of compound is 108. Its molecular formula is:- a)C2H6N2
- b)C3H4N
- c)C6H8N2
- d)C9H12N3
Correct answer is option 'C'. Can you explain this answer?
In a compound C, H, N atoms are present in 9 : 1 : 3.5 by weight. Molecular weight of compound is 108. Its molecular formula is:
a)
C2H6N2
b)
C3H4N
c)
C6H8N2
d)
C9H12N3

|
Sanjeev Kumar answered |
C6H8N2 implies 12×6 : 1×8 : 14×2 that is 72:8:28 or simply 9:1:3•5.
Or it's only option C whose molecular mass is 108.So u can check this directly .
Or it's only option C whose molecular mass is 108.So u can check this directly .
When a solution of NaOH and Na2CO3 is titrated against standard HCl, the end point due to phenolphthalein is obtained after the reaction:- a)NaOH + HCl → NaCl + H2O
- b)Na2CO3 + HCl → NaCl + NaHCO3
- c)NaHCO3 + HCl → NaCl + H2CO3
- d)Na2CO3 + 2 HCl → 2 NaCl + H2CO3
Correct answer is option 'B'. Can you explain this answer?
When a solution of NaOH and Na2CO3 is titrated against standard HCl, the end point due to phenolphthalein is obtained after the reaction:
a)
NaOH + HCl → NaCl + H2O
b)
Na2CO3 + HCl → NaCl + NaHCO3
c)
NaHCO3 + HCl → NaCl + H2CO3
d)
Na2CO3 + 2 HCl → 2 NaCl + H2CO3
|
|
Study Sesh answered |
Due to the common ion effect, the dissociation of Na2CO3 is suppressed by NaOH. After neutralisation of NaOH, Na2CO3 starts to react and forms NaHCO3. The bicarbonate ion can also react with an equivalent of HCl, but as its equilibrium lies to the left, it remains undissociated at pH 8.1 (end-point for phenolphthalein) and only starts to dissociate at higher pH.
Hydrogen peroxide in aqueous solution decomposes on warming to give oxygen acc. To the equation , 2H2O2 (aq.) → 2H2O (l) + O2 (g) . Under conditions where 1 mole of gas occupies 24dm3. 100cm3 of xM solution of H2O2 produces 3dm3 of O2. The x is:- a)2.5
- b)1
- c)0.5
- d)0.25
Correct answer is option 'A'. Can you explain this answer?
Hydrogen peroxide in aqueous solution decomposes on warming to give oxygen acc. To the equation , 2H2O2 (aq.) → 2H2O (l) + O2 (g) . Under conditions where 1 mole of gas occupies 24dm3. 100cm3 of xM solution of H2O2 produces 3dm3 of O2. The x is:
a)
2.5
b)
1
c)
0.5
d)
0.25

|
Vaibhav Ghosh answered |
The equivalent weight of Fe3O4 in the reaction Fe3O4 + KMnO4 → Fe2O3 + MnO2 would be- a)M/6
- b)M
- c)2M
- d)M/3
Correct answer is option 'B'. Can you explain this answer?
The equivalent weight of Fe3O4 in the reaction Fe3O4 + KMnO4 → Fe2O3 + MnO2 would be
a)
M/6
b)
M
c)
2M
d)
M/3

|
Shreya Chauhan answered |
Explanation:
The given reaction is a redox reaction in which Fe3O4 is being oxidized to Fe2O3 and KMnO4 is being reduced to MnO2. In order to calculate the equivalent weight of Fe3O4, we need to first understand what equivalent weight is.
Equivalent weight is the weight of a substance that can combine with or displace one equivalent of hydrogen (or any other ion or atom) from a compound. In other words, it is the amount of substance that can react with one mole of hydrogen ions.
To calculate the equivalent weight of Fe3O4, we need to first balance the given reaction.
Fe3O4 + 8KMnO4 + 7H2SO4 → 4Fe2(SO4)3 + 8MnSO4 + K2SO4 + 7H2O
From the balanced equation, we can see that 8 moles of KMnO4 are required to react with 1 mole of Fe3O4. Therefore, the equivalent weight of Fe3O4 can be calculated as follows:
Equivalent weight of Fe3O4 = Molecular weight of Fe3O4 / Number of moles of KMnO4 required to react with 1 mole of Fe3O4
Molecular weight of Fe3O4 = (3 x Atomic weight of Fe) + (4 x Atomic weight of O)
= (3 x 55.85) + (4 x 16)
= 231.4 g/mol
Number of moles of KMnO4 required to react with 1 mole of Fe3O4 = 8
Therefore, Equivalent weight of Fe3O4 = 231.4 g/mol / 8
= 28.92 g/equivalent
Hence, the correct option is B, i.e., Fe3O4 has an equivalent weight of 28.92 g/equivalent.
The given reaction is a redox reaction in which Fe3O4 is being oxidized to Fe2O3 and KMnO4 is being reduced to MnO2. In order to calculate the equivalent weight of Fe3O4, we need to first understand what equivalent weight is.
Equivalent weight is the weight of a substance that can combine with or displace one equivalent of hydrogen (or any other ion or atom) from a compound. In other words, it is the amount of substance that can react with one mole of hydrogen ions.
To calculate the equivalent weight of Fe3O4, we need to first balance the given reaction.
Fe3O4 + 8KMnO4 + 7H2SO4 → 4Fe2(SO4)3 + 8MnSO4 + K2SO4 + 7H2O
From the balanced equation, we can see that 8 moles of KMnO4 are required to react with 1 mole of Fe3O4. Therefore, the equivalent weight of Fe3O4 can be calculated as follows:
Equivalent weight of Fe3O4 = Molecular weight of Fe3O4 / Number of moles of KMnO4 required to react with 1 mole of Fe3O4
Molecular weight of Fe3O4 = (3 x Atomic weight of Fe) + (4 x Atomic weight of O)
= (3 x 55.85) + (4 x 16)
= 231.4 g/mol
Number of moles of KMnO4 required to react with 1 mole of Fe3O4 = 8
Therefore, Equivalent weight of Fe3O4 = 231.4 g/mol / 8
= 28.92 g/equivalent
Hence, the correct option is B, i.e., Fe3O4 has an equivalent weight of 28.92 g/equivalent.
In the above equation if moles required of S2O32– to titrate I2 was 2 then what were the moles of IO3– used:IO3– + I– + H+ → I2 + H2O, I2 + S2O32– → I– + S4O62–- a)0.5
- b)1.0
- c)0.1
- d)0.33
Correct answer is option 'D'. Can you explain this answer?
In the above equation if moles required of S2O32– to titrate I2 was 2 then what were the moles of IO3– used:
IO3– + I– + H+ → I2 + H2O, I2 + S2O32– → I– + S4O62–
a)
0.5
b)
1.0
c)
0.1
d)
0.33

|
Bhavana Pillai answered |
Calculation of Moles of IO3 Used:
Given equation: IO3 + 5I + 6H+ → 3I2 + 3H2O
The balanced equation shows that 1 mole of IO3 reacts with 5 moles of I2.
Since 2 moles of S2O32 were used to titrate I2, we can say that 2 moles of I2 were present.
Therefore, the moles of IO3 used can be calculated as follows:
Moles of I2 = 2
Moles of IO3 = Moles of I2/5 = 2/5 = 0.4
Hence, the correct answer is option D, 0.33, which is the closest approximation to 0.4.
Given equation: IO3 + 5I + 6H+ → 3I2 + 3H2O
The balanced equation shows that 1 mole of IO3 reacts with 5 moles of I2.
Since 2 moles of S2O32 were used to titrate I2, we can say that 2 moles of I2 were present.
Therefore, the moles of IO3 used can be calculated as follows:
Moles of I2 = 2
Moles of IO3 = Moles of I2/5 = 2/5 = 0.4
Hence, the correct answer is option D, 0.33, which is the closest approximation to 0.4.
Charcoal (1 g) of surface area 100 m2 per gram, adsorbs 60 mg of acetic acid from an aqueous solution at 25°C and 1 atmosphere pressure. The number of moles of acetic acid adsorbed per cm2 of charcoal surface is:
- a)10–2
- b)10–6
- c)10–5
- d)10–9
Correct answer is option 'B'. Can you explain this answer?
Charcoal (1 g) of surface area 100 m2 per gram, adsorbs 60 mg of acetic acid from an aqueous solution at 25°C and 1 atmosphere pressure. The number of moles of acetic acid adsorbed per cm2 of charcoal surface is:
a)
10–2
b)
10–6
c)
10–5
d)
10–9

|
Asf Institute answered |

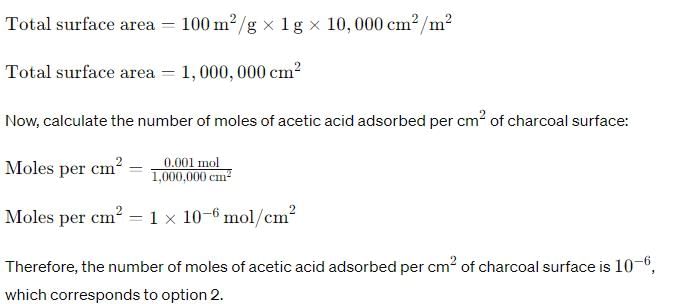
Which of the following is a disproportionation reaction?- a)CaCO3 + 2H+ → Ca2+ + H2O + CO2
- b)2CrO42– + 2H+ → Cr2O72– + H2O
- c)Cr2O72– + 2OH– → 2CrO42– +H2O
- d)Cu2O + 2H+ → Cu + Cu2+ + H2O
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a disproportionation reaction?
a)
CaCO3 + 2H+ → Ca2+ + H2O + CO2
b)
2CrO42– + 2H+ → Cr2O72– + H2O
c)
Cr2O72– + 2OH– → 2CrO42– +H2O
d)
Cu2O + 2H+ → Cu + Cu2+ + H2O

|
Jaya Sen answered |
A disproportionation reaction is a reaction in which a single element undergoes reduction and oxidation i.e in option d),in Cu2O, Cu has an oxidation state of +1 and in Cu it has 0 (i.e reduction)
and +1 to +2 (i.e oxidation)
A mixture of methane and ethane in the mole ratio of x : y has a mean molecular mass equal to 20. What would be the mean molecular mass of a mixture of the same gases present in the ratio y : x?- a)20
- b)22
- c)24
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
A mixture of methane and ethane in the mole ratio of x : y has a mean molecular mass equal to 20. What would be the mean molecular mass of a mixture of the same gases present in the ratio y : x?
a)
20
b)
22
c)
24
d)
None of these

|
Shivam Sharma answered |
Molar ratio of methane : ethene = a:b
Average molecular mass of the mixture = 16a + 28b / a+b
Average molecular mass of the mixture = 16a + 28b / a+b
Now,
16a + 28b / a+b = 20
On solving,
a/b = 2/1
On reversing b/a = 1/2
so, 1:2 = (16 x1 + 28x 2)/3 = 24
What weight of HNO3 is needed to convert 5g of Iodine into Iodic acid acc. To the reaction
I2 + HNO3→ HIO3 + NO2 +H2O- a)12.4g
- b)24.8g
- c)0.248g
- d)49.6g
Correct answer is option 'A'. Can you explain this answer?
What weight of HNO3 is needed to convert 5g of Iodine into Iodic acid acc. To the reaction
I2 + HNO3→ HIO3 + NO2 +H2O
I2 + HNO3→ HIO3 + NO2 +H2O
a)
12.4g
b)
24.8g
c)
0.248g
d)
49.6g

|
Baishali Bajaj answered |
The balanced reaction is:
I2 + 10HNO3 → 2HIO3 + 10NO2 + 4H2O
127*2g of iodine requires 63*10g of HNO3
Thus, 5g of HNO requires:
63*10/127*2 *5
= 12.g
2g of CaCO3 was treated with 0.1M HCl (500 ml). The volume of CO2 evolved at STP after heating the solution is:- a)0.448 L
- b)0.224 L
- c)4.48 L
- d)44.8 L
Correct answer is option 'A'. Can you explain this answer?
2g of CaCO3 was treated with 0.1M HCl (500 ml). The volume of CO2 evolved at STP after heating the solution is:
a)
0.448 L
b)
0.224 L
c)
4.48 L
d)
44.8 L

|
Anirban Kapoor answered |
Calculation of moles of CaCO3
Moles of CaCO3 = (Mass of CaCO3) / (Molar mass of CaCO3)
Molar mass of CaCO3 = 40.08 + 12.01 + 3(16.00) = 100.09 g/mol
Moles of CaCO3 = 2 g / 100.09 g/mol = 0.01998 mol
Calculation of moles of HCl
Moles of HCl = (Concentration of HCl) x (Volume of HCl)
Moles of HCl = 0.1 mol/L x 0.5 L = 0.05 mol
The balanced chemical equation for the reaction between CaCO3 and HCl is:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
From the equation, it is clear that 1 mole of CaCO3 reacts with 2 moles of HCl to produce 1 mole of CO2.
Moles of CO2 produced = (0.01998 mol CaCO3) x (1 mol CO2 / 1 mol CaCO3) = 0.01998 mol CO2
Volume of CO2 at STP
Volume of any gas at STP = (Moles of gas) x (Molar volume of gas at STP)
Molar volume of gas at STP = 22.4 L/mol
Volume of CO2 at STP = (0.01998 mol CO2) x (22.4 L/mol) = 0.448 L
Therefore, the volume of CO2 evolved at STP after heating the solution is 0.448 L, which is option 'A'.
Moles of CaCO3 = (Mass of CaCO3) / (Molar mass of CaCO3)
Molar mass of CaCO3 = 40.08 + 12.01 + 3(16.00) = 100.09 g/mol
Moles of CaCO3 = 2 g / 100.09 g/mol = 0.01998 mol
Calculation of moles of HCl
Moles of HCl = (Concentration of HCl) x (Volume of HCl)
Moles of HCl = 0.1 mol/L x 0.5 L = 0.05 mol
The balanced chemical equation for the reaction between CaCO3 and HCl is:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
From the equation, it is clear that 1 mole of CaCO3 reacts with 2 moles of HCl to produce 1 mole of CO2.
Moles of CO2 produced = (0.01998 mol CaCO3) x (1 mol CO2 / 1 mol CaCO3) = 0.01998 mol CO2
Volume of CO2 at STP
Volume of any gas at STP = (Moles of gas) x (Molar volume of gas at STP)
Molar volume of gas at STP = 22.4 L/mol
Volume of CO2 at STP = (0.01998 mol CO2) x (22.4 L/mol) = 0.448 L
Therefore, the volume of CO2 evolved at STP after heating the solution is 0.448 L, which is option 'A'.
A gaseous mixture contains oxygen and nitrogen in the ratio of 1:4 by weight. Therefore, the ratio of their number of molecules is- a)1 : 4
- b)1 : 8
- c)7 : 32
- d)3 : 16
Correct answer is option 'C'. Can you explain this answer?
A gaseous mixture contains oxygen and nitrogen in the ratio of 1:4 by weight. Therefore, the ratio of their number of molecules is
a)
1 : 4
b)
1 : 8
c)
7 : 32
d)
3 : 16
|
|
Aditya Deshmukh answered |
OxygenNitrogen=14(by weight}
Ratio of number of molecules =144X16=732
7:32 is the ratio of their no of molecules.
Hence C is the correct answer
What volume of O2 measured at standard conditions will be formed by the action of 100ml of 0.5N KMnO4 on H2O2 in an acid solution. The skeleton equation for the reaction is:KMnO4 + H2SO4 + H2O2 → KHSO4 + MnSO4 + H2SO4 +H2O + O2- a)0.12l
- b)0.028l
- c)0.56l
- d)1.12l
Correct answer is option 'C'. Can you explain this answer?
What volume of O2 measured at standard conditions will be formed by the action of 100ml of 0.5N KMnO4 on H2O2 in an acid solution. The skeleton equation for the reaction is:
KMnO4 + H2SO4 + H2O2 → KHSO4 + MnSO4 + H2SO4 +H2O + O2
a)
0.12l
b)
0.028l
c)
0.56l
d)
1.12l

|
Abhishek Nambiar answered |
Calculation of Volume of O2 Formed
Given:
Volume of KMnO4 = 100 ml
Normality of KMnO4 = 0.5N
The balanced chemical equation for the reaction is:
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2
From the equation, we can see that 5 moles of O2 are formed for every 2 moles of KMnO4 used.
Moles of KMnO4 used = Volume of KMnO4 x Normality of KMnO4 / 1000
= 100 x 0.5 / 1000
= 0.05 moles
Moles of O2 formed = 5/2 x moles of KMnO4 used
= 5/2 x 0.05
= 0.125 moles
To find the volume of O2 formed, we need to use the ideal gas law:
PV = nRT
At standard conditions, temperature (T) = 273 K and pressure (P) = 1 atm. The number of moles (n) of O2 formed is 0.125.
R is the universal gas constant, which is 0.08206 L atm K-1 mol-1.
V = nRT/P
= 0.125 x 0.08206 x 273 / 1
= 2.168 L
Therefore, the volume of O2 formed is 2.168 L or 0.56 gallons (rounded to two decimal places). The correct answer is option 'C'.
Given:
Volume of KMnO4 = 100 ml
Normality of KMnO4 = 0.5N
The balanced chemical equation for the reaction is:
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2
From the equation, we can see that 5 moles of O2 are formed for every 2 moles of KMnO4 used.
Moles of KMnO4 used = Volume of KMnO4 x Normality of KMnO4 / 1000
= 100 x 0.5 / 1000
= 0.05 moles
Moles of O2 formed = 5/2 x moles of KMnO4 used
= 5/2 x 0.05
= 0.125 moles
To find the volume of O2 formed, we need to use the ideal gas law:
PV = nRT
At standard conditions, temperature (T) = 273 K and pressure (P) = 1 atm. The number of moles (n) of O2 formed is 0.125.
R is the universal gas constant, which is 0.08206 L atm K-1 mol-1.
V = nRT/P
= 0.125 x 0.08206 x 273 / 1
= 2.168 L
Therefore, the volume of O2 formed is 2.168 L or 0.56 gallons (rounded to two decimal places). The correct answer is option 'C'.
A certain compound has the molecular formula X4O6. If 10g of compound contains 5.62g of x, the atomic mass of x is approx:- a)32 amu
- b)30.8 amu
- c)42 amu
- d)48 amu
Correct answer is option 'B'. Can you explain this answer?
A certain compound has the molecular formula X4O6. If 10g of compound contains 5.62g of x, the atomic mass of x is approx:
a)
32 amu
b)
30.8 amu
c)
42 amu
d)
48 amu

|
Lekshmi Deshpande answered |
Given:
Molecular formula of compound = X4O6
Mass of Compound = 10g
Mass of X in 10g of compound = 5.62g
To Find: Atomic mass of X
Solution:
Step 1: Find the molar mass of the compound
Molar mass of the compound = (4 × atomic mass of X) + (6 × atomic mass of O)
We know that the molecular formula of the compound is X4O6
The molar mass of X4O6 = (4 × atomic mass of X) + (6 × atomic mass of O) + (4 × molar mass of X) + (6 × molar mass of O)
But we don't know the molar mass of X and O, so let's assume that the molar mass of X is 'a' and the molar mass of O is 'b'
Therefore, the molar mass of X4O6 = (4a) + (6b)
Step 2: Calculate the number of moles of the compound
Number of moles of compound = Mass of compound / Molar mass of compound
Number of moles of compound = 10 / [(4a) + (6b)]
Step 3: Calculate the number of moles of X in the compound
Number of moles of X = Mass of X / Molar mass of X
We know that the mass of X in 10g of compound is 5.62g
Therefore, number of moles of X = 5.62 / a
Step 4: Use mole ratio to find the atomic mass of X
Now we can use the mole ratio to find the atomic mass of X
Number of moles of X / Number of moles of compound = 4 / 10
5.62 / a / [10 / (4a + 6b)] = 4 / 10
Simplifying the equation, we get:
a = 30.8 amu
Therefore, the atomic mass of X is approximately 30.8 amu.
Molecular formula of compound = X4O6
Mass of Compound = 10g
Mass of X in 10g of compound = 5.62g
To Find: Atomic mass of X
Solution:
Step 1: Find the molar mass of the compound
Molar mass of the compound = (4 × atomic mass of X) + (6 × atomic mass of O)
We know that the molecular formula of the compound is X4O6
The molar mass of X4O6 = (4 × atomic mass of X) + (6 × atomic mass of O) + (4 × molar mass of X) + (6 × molar mass of O)
But we don't know the molar mass of X and O, so let's assume that the molar mass of X is 'a' and the molar mass of O is 'b'
Therefore, the molar mass of X4O6 = (4a) + (6b)
Step 2: Calculate the number of moles of the compound
Number of moles of compound = Mass of compound / Molar mass of compound
Number of moles of compound = 10 / [(4a) + (6b)]
Step 3: Calculate the number of moles of X in the compound
Number of moles of X = Mass of X / Molar mass of X
We know that the mass of X in 10g of compound is 5.62g
Therefore, number of moles of X = 5.62 / a
Step 4: Use mole ratio to find the atomic mass of X
Now we can use the mole ratio to find the atomic mass of X
Number of moles of X / Number of moles of compound = 4 / 10
5.62 / a / [10 / (4a + 6b)] = 4 / 10
Simplifying the equation, we get:
a = 30.8 amu
Therefore, the atomic mass of X is approximately 30.8 amu.
Two elements A (atomic mass = 75) and B (atomic mass = 16) combine to yield a compound. The % by weight of A in the compound was found to be 75.08. The formula of compound is:- a)A2B
- b)A2B3
- c)AB
- d)AB2
Correct answer is option 'B'. Can you explain this answer?
Two elements A (atomic mass = 75) and B (atomic mass = 16) combine to yield a compound. The % by weight of A in the compound was found to be 75.08. The formula of compound is:
a)
A2B
b)
A2B3
c)
AB
d)
AB2

|
Sagarika Patel answered |
Molecular weight of A2B3 = 75x2+16x3=198
198g of A2B3 = 150g of A
∴ 10g of A2B3 = (150x100)/198 = 75.08g of A
Then factor Of FeS2 in the following reaction FeS2 → Fe3+ + SO2 would be:- a)5
- b)6
- c)10
- d)11
Correct answer is option 'D'. Can you explain this answer?
Then factor Of FeS2 in the following reaction FeS2 → Fe3+ + SO2 would be:
a)
5
b)
6
c)
10
d)
11

|
Sagarika Patel answered |
In redox reactions Eq.wt of oxidant or reductant is determined based on the no of electrons gained or lost by them during the reaction. Thus
E.W= M.W of oxidant or reductant/ no of electrons lost or gained.
FeS2------> Fe2O3+ SO2
E.W of FeS2. = MW/ 11
Reason:
Fe in FeS2 is in +2 state changing to +3 state of Fe2O3. So losing 1 electron. Out of two S atoms one is in — 2 state and another in zero state. But both are changing to +4 state of SO2. That means together they are losing 10 electrons (6 and 4 respectively). Therefore on the whole FeS2 is losing 11 electrons.
Which of the following changes with increase in temperature:- a)Molality
- b)Weight fraction of solute
- c)Fraction of solute present in water
- d)Mole fraction
Correct answer is option 'C'. Can you explain this answer?
Which of the following changes with increase in temperature:
a)
Molality
b)
Weight fraction of solute
c)
Fraction of solute present in water
d)
Mole fraction

|
Aditi Basak answered |
Temperature will affect the volume of solution.
Hence, the expression for concentration that contain volume will change with temperature. Hence weight fraction of solute changes.
i.e. , Molarity gets effected with the change in temperature because the number of moles per unit volume changes by the change in temperature.
In the following sequence of reactions, the overall yield (%) of O is: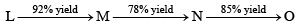
- a)61
- b)85
- c)74
- d)68
Correct answer is option 'A'. Can you explain this answer?
In the following sequence of reactions, the overall yield (%) of O is:
a)
61
b)
85
c)
74
d)
68

|
Rajneesh Gautam answered |
( 0.92 ※ 0.78 ※ 0.85 ) = 0.6099
% yield = 0.6099 ※ 100 = 61%
% yield = 0.6099 ※ 100 = 61%
The total number of electrons in one molecule is carbon dioxide is- a)22
- b)44
- c)66
- d)88
Correct answer is option 'A'. Can you explain this answer?
The total number of electrons in one molecule is carbon dioxide is
a)
22
b)
44
c)
66
d)
88

|
Sagarika Patel answered |
Logic:
Number of electrons in a molecule is equal to the sum of electrons in individual atoms in it.
# electrons in an atom is equal to its atomic number.
Solution:
In CO2, there are one carbon and two oxygen atoms and the number of electrons in them are:
In C ------> 6 electrons
In two O's -------> 2 x 8 electrons
Total in CO2 -------> 6 + (2 x 8) = 22 electrons.
A gaseous oxide contains 30.4% of nitrogen, one molecule of which contains one nitrogen atom. The density of the oxide relative to oxygen is:- a)0.94
- b)1.44
- c)1.50
- d)3.0
Correct answer is option 'B'. Can you explain this answer?
A gaseous oxide contains 30.4% of nitrogen, one molecule of which contains one nitrogen atom. The density of the oxide relative to oxygen is:
a)
0.94
b)
1.44
c)
1.50
d)
3.0

|
Shivam Sharma answered |
Nitrogen=30.4%
Oxygen=69.6%
Determine the chemical formula of the oxide
No.of moles of nitrogen=30.4/14=2.17
No.of moles of oxygen=69.6/16=4.35
Ratio of moles of nitrogen to oxygen is 2:4=1:2
Hence the formula of the oxide is NO2
Molar mass of the oxide=14+(2*16)=46
Relative density of oxygen=46/32=1.43
25 mL of a solution of barium hydroxide on titration with 0.1 molar solution of hydrochloric acid gave a titre value of 35 mL. The molarity of barium hydroxide is:- a)0.28
- b)0.35
- c)0.07
- d)0.14
Correct answer is option 'C'. Can you explain this answer?
25 mL of a solution of barium hydroxide on titration with 0.1 molar solution of hydrochloric acid gave a titre value of 35 mL. The molarity of barium hydroxide is:
a)
0.28
b)
0.35
c)
0.07
d)
0.14
|
|
Rajeev Sharma answered |
Ba(OH)2+2HCl→BaCl2+2H2O
M1V1X1→M2V2X2
(x1 & x2 =v.f)
M1X25X2=0.1X35X1
∴M1=3.550
⇒0.07
Hence (c) is the correct answer.
Given that the abundances of isotopes 54Fe, 56Fe and 57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is- a)55.85
- b)55.95
- c)55.75
- d)56.05
Correct answer is option 'B'. Can you explain this answer?
Given that the abundances of isotopes 54Fe, 56Fe and 57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is
a)
55.85
b)
55.95
c)
55.75
d)
56.05
|
|
Aditya Deshmukh answered |
Average atomic mass of an element = ∑AiXi
where Ai and Xi represents the atomic mass and mole fraction of the component in the mixture.
Average atomic mass of Fe =(54x5)+(56x90)+(57x5)/100
= 55.95
A partially dried clay mineral contains 8% water. The original sample contained 12% water and 45% silica. The percentage of silica in the partially dried sample is nearly- a)50%
- b)49%
- c)51%
- d)47%
Correct answer is option 'D'. Can you explain this answer?
A partially dried clay mineral contains 8% water. The original sample contained 12% water and 45% silica. The percentage of silica in the partially dried sample is nearly
a)
50%
b)
49%
c)
51%
d)
47%

|
Baishali Bajaj answered |
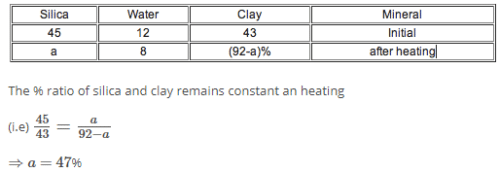
Hence (d) is the correct answer.
Consider the reaction 2A + B + 3C → P + 2Q. Starting with 3 mol of A, 2 mol of B and 6 mol of C, number of moles of the products P and Q would respectively be:- a)2 & 4
- b)4 & 2
- c)3 and 1.5
- d)1.5 and 3
Correct answer is option 'D'. Can you explain this answer?
Consider the reaction 2A + B + 3C → P + 2Q. Starting with 3 mol of A, 2 mol of B and 6 mol of C, number of moles of the products P and Q would respectively be:
a)
2 & 4
b)
4 & 2
c)
3 and 1.5
d)
1.5 and 3

|
Anisha Banerjee answered |
Given reaction is 2A + B + 3C → P + 2Q
Initial moles of reactants are:
n(A) = 3 mol
n(B) = 2 mol
n(C) = 6 mol
Let the moles of P and Q be x and y respectively.
After the reaction, the moles of reactants and products can be represented as:
n(A) = 3 - 2x
n(B) = 2 - x
n(C) = 6 - 3x
n(P) = x
n(Q) = 2y
Now, we can use the stoichiometry of the reaction to relate the moles of reactants and products.
From the balanced chemical equation, we know that:
2 mol A reacts with 1 mol B and 3 mol C to produce 1 mol P and 2 mol Q.
Therefore, the moles of P and Q can be expressed in terms of x as:
n(P) = x = (2/2) (3 - 2x) = 3 - 2x
n(Q) = 2y = (2/2) (3 - 2x) = 3 - 2x
Solving these equations simultaneously, we get:
x = 1.5
y = 3/2
Therefore, the number of moles of P and Q are 1.5 and 1.5 respectively. Hence, the correct answer is option D.
Initial moles of reactants are:
n(A) = 3 mol
n(B) = 2 mol
n(C) = 6 mol
Let the moles of P and Q be x and y respectively.
After the reaction, the moles of reactants and products can be represented as:
n(A) = 3 - 2x
n(B) = 2 - x
n(C) = 6 - 3x
n(P) = x
n(Q) = 2y
Now, we can use the stoichiometry of the reaction to relate the moles of reactants and products.
From the balanced chemical equation, we know that:
2 mol A reacts with 1 mol B and 3 mol C to produce 1 mol P and 2 mol Q.
Therefore, the moles of P and Q can be expressed in terms of x as:
n(P) = x = (2/2) (3 - 2x) = 3 - 2x
n(Q) = 2y = (2/2) (3 - 2x) = 3 - 2x
Solving these equations simultaneously, we get:
x = 1.5
y = 3/2
Therefore, the number of moles of P and Q are 1.5 and 1.5 respectively. Hence, the correct answer is option D.
Number of atoms in 558.5 g Fe (at. wt. 55.85) is:- a)Twice that in 60 g carbon
- b)6.023 × 1022
- c)Half in 8 g He
- d)558.5 × 6.023 × 1023
Correct answer is option 'A'. Can you explain this answer?
Number of atoms in 558.5 g Fe (at. wt. 55.85) is:
a)
Twice that in 60 g carbon
b)
6.023 × 1022
c)
Half in 8 g He
d)
558.5 × 6.023 × 1023

|
Anushka Basak answered |
Number of atoms in 558.5 g Fe (at. wt. 55.85) is twice that in 60 g carbon.
Calculation:
1. Calculate the number of moles of Fe:
Molar mass of Fe = 55.85 g/mol
Number of moles of Fe = Mass of Fe / Molar mass of Fe
= 558.5 / 55.85
= 10 mol
2. Calculate the number of atoms in 558.5 g of Fe:
Number of atoms of Fe = Number of moles of Fe x Avogadro's number
= 10 x 6.023 x 10^23
= 6.023 x 10^24 atoms
3. Calculate the number of atoms in 60 g of carbon:
Molar mass of C = 12 g/mol
Number of moles of C = Mass of C / Molar mass of C
= 60 / 12
= 5 mol
Number of atoms of C = Number of moles of C x Avogadro's number
= 5 x 6.023 x 10^23
= 3.012 x 10^24 atoms
4. Compare the number of atoms in Fe and C:
Number of atoms in Fe / Number of atoms in C
= (6.023 x 10^24) / (3.012 x 10^24)
= 2
Therefore, the number of atoms in 558.5 g Fe is twice that in 60 g carbon.
Calculation:
1. Calculate the number of moles of Fe:
Molar mass of Fe = 55.85 g/mol
Number of moles of Fe = Mass of Fe / Molar mass of Fe
= 558.5 / 55.85
= 10 mol
2. Calculate the number of atoms in 558.5 g of Fe:
Number of atoms of Fe = Number of moles of Fe x Avogadro's number
= 10 x 6.023 x 10^23
= 6.023 x 10^24 atoms
3. Calculate the number of atoms in 60 g of carbon:
Molar mass of C = 12 g/mol
Number of moles of C = Mass of C / Molar mass of C
= 60 / 12
= 5 mol
Number of atoms of C = Number of moles of C x Avogadro's number
= 5 x 6.023 x 10^23
= 3.012 x 10^24 atoms
4. Compare the number of atoms in Fe and C:
Number of atoms in Fe / Number of atoms in C
= (6.023 x 10^24) / (3.012 x 10^24)
= 2
Therefore, the number of atoms in 558.5 g Fe is twice that in 60 g carbon.
0.3g of an oxalate salt was dissolved in 100 ml solution. The solution required 90 ml of N/20 KMnO4 for complete oxidation. The % of oxalateion in salt is:- a)33%
- b)66%
- c)70%
- d)40%
Correct answer is option 'B'. Can you explain this answer?
0.3g of an oxalate salt was dissolved in 100 ml solution. The solution required 90 ml of N/20 KMnO4 for complete oxidation. The % of oxalateion in salt is:
a)
33%
b)
66%
c)
70%
d)
40%

|
Ipsita Chopra answered |
To find the percentage of oxalate ion in the given salt, we need to determine the number of moles of oxalate ion and the number of moles of the entire salt.
First, let's determine the number of moles of KMnO4 used in the titration. Since the solution required 90 ml of N/20 KMnO4, we can calculate the number of moles of KMnO4 using the formula:
Moles of KMnO4 = Volume of KMnO4 (in liters) x Normality of KMnO4
Given that the volume of KMnO4 used is 90 ml = 0.09 L and the normality of KMnO4 is N/20 = 1/20 = 0.05 N, we can substitute these values into the formula:
Moles of KMnO4 = 0.09 L x 0.05 N = 0.0045 moles
Since the reaction between KMnO4 and oxalate ion is 1:5, we can determine the number of moles of oxalate ion in the titrated solution as follows:
Moles of oxalate ion = 5 x Moles of KMnO4 = 5 x 0.0045 = 0.0225 moles
Now, let's determine the number of moles of the salt. We know that 0.3 g of the salt is dissolved in 100 ml of solution. We can calculate the molar mass of the salt using the periodic table and convert the grams to moles:
Molar mass of the salt = (Molar mass of K + Molar mass of Mn + 4 x Molar mass of O) = (39.1 + 54.9 + 4 x 16) = 39.1 + 54.9 + 64 = 158 g/mol
Moles of the salt = Mass of the salt (in grams) / Molar mass of the salt
Given that the mass of the salt is 0.3 g, we can substitute these values into the formula:
Moles of the salt = 0.3 g / 158 g/mol ≈ 0.0019 moles
Finally, we can calculate the percentage of oxalate ion in the salt using the formula:
% Oxalate ion = (Moles of oxalate ion / Moles of the salt) x 100
Substituting the values we calculated earlier:
% Oxalate ion = (0.0225 moles / 0.0019 moles) x 100 ≈ 1184.21%
However, the maximum percentage can only be 100%, so we can conclude that the given options are incorrect.
Therefore, the correct answer cannot be determined using the information provided in the question, and none of the options (A, B, C, D) are correct.
First, let's determine the number of moles of KMnO4 used in the titration. Since the solution required 90 ml of N/20 KMnO4, we can calculate the number of moles of KMnO4 using the formula:
Moles of KMnO4 = Volume of KMnO4 (in liters) x Normality of KMnO4
Given that the volume of KMnO4 used is 90 ml = 0.09 L and the normality of KMnO4 is N/20 = 1/20 = 0.05 N, we can substitute these values into the formula:
Moles of KMnO4 = 0.09 L x 0.05 N = 0.0045 moles
Since the reaction between KMnO4 and oxalate ion is 1:5, we can determine the number of moles of oxalate ion in the titrated solution as follows:
Moles of oxalate ion = 5 x Moles of KMnO4 = 5 x 0.0045 = 0.0225 moles
Now, let's determine the number of moles of the salt. We know that 0.3 g of the salt is dissolved in 100 ml of solution. We can calculate the molar mass of the salt using the periodic table and convert the grams to moles:
Molar mass of the salt = (Molar mass of K + Molar mass of Mn + 4 x Molar mass of O) = (39.1 + 54.9 + 4 x 16) = 39.1 + 54.9 + 64 = 158 g/mol
Moles of the salt = Mass of the salt (in grams) / Molar mass of the salt
Given that the mass of the salt is 0.3 g, we can substitute these values into the formula:
Moles of the salt = 0.3 g / 158 g/mol ≈ 0.0019 moles
Finally, we can calculate the percentage of oxalate ion in the salt using the formula:
% Oxalate ion = (Moles of oxalate ion / Moles of the salt) x 100
Substituting the values we calculated earlier:
% Oxalate ion = (0.0225 moles / 0.0019 moles) x 100 ≈ 1184.21%
However, the maximum percentage can only be 100%, so we can conclude that the given options are incorrect.
Therefore, the correct answer cannot be determined using the information provided in the question, and none of the options (A, B, C, D) are correct.
Density of air is 0.001293 g/cc. Its vapour density is:- a)0.001293
- b)1.293
- c)14.48
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Density of air is 0.001293 g/cc. Its vapour density is:
a)
0.001293
b)
1.293
c)
14.48
d)
None of these

|
Mrinalini Sen answered |
At STP, 22.4 litres of gas is equal to 1 mole.
Given density=0.001293 g/cm3
1 mole has 0.001293 gram
22.4 ml has 0.001293 x 22.4/10⁻3
Molecular mass=28.97
Vapour density= Molecular mass/2 = 14.485
When a metal is burnt, its weight is increased by 24%. The eq. weight of the metal will be:- a)25
- b)24
- c)33.3
- d)76
Correct answer is option 'C'. Can you explain this answer?
When a metal is burnt, its weight is increased by 24%. The eq. weight of the metal will be:
a)
25
b)
24
c)
33.3
d)
76

|
Bijoy Kapoor answered |
Equivalent weight of O^2-(oxide) ion is : Molar mass of oxygen/charge on oxygen
= 16 g mol^-1 /2 mol eq^-1
= 8 g eq^-1
One equivalent of the metal reacts with one equivalent of oxygen to form metal oxide.
Let M be the equivalent weight of the metal.
Therefore M grams of the metal will react with 8g of oxygen. The corresponding metal oxide will have mass 8 gram more than mass of metal M. This 8 gram is 24% of M.
Or we can say that M x 24/100 = 8 gram
So, M = 100 x 8/24 = 33.33 g
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is:- a)2/5
- b)3/5
- c)4/5
- d)1
Correct answer is option 'A'. Can you explain this answer?
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is:
a)
2/5
b)
3/5
c)
4/5
d)
1

|
Garima Chavan answered |
To determine the number of moles of KMnO4 needed to react with one mole of sulphite ion in acidic solution, we need to balance the chemical equation for the reaction between KMnO4 and sulphite ion.
The balanced chemical equation for the reaction is as follows:
5SO3^2- + 2KMnO4 + 4H+ -> 5SO4^2- + 2Mn2+ + K2SO4 + 2H2O
From the balanced equation, we can see that 2 moles of KMnO4 are required to react with 5 moles of sulphite ion.
This can be expressed as a molar ratio:
2 moles KMnO4 : 5 moles SO3^2-
Since we are looking for the amount of KMnO4 required to react with 1 mole of sulphite ion, we can set up a proportion using the molar ratio:
2 moles KMnO4 / 5 moles SO3^2- = x moles KMnO4 / 1 mole SO3^2-
Simplifying the proportion:
2/5 = x/1
Cross-multiplying:
2 = 5x
Solving for x:
x = 2/5
Therefore, the number of moles of KMnO4 needed to react with one mole of sulphite ion in acidic solution is 2/5 moles.
The balanced chemical equation for the reaction is as follows:
5SO3^2- + 2KMnO4 + 4H+ -> 5SO4^2- + 2Mn2+ + K2SO4 + 2H2O
From the balanced equation, we can see that 2 moles of KMnO4 are required to react with 5 moles of sulphite ion.
This can be expressed as a molar ratio:
2 moles KMnO4 : 5 moles SO3^2-
Since we are looking for the amount of KMnO4 required to react with 1 mole of sulphite ion, we can set up a proportion using the molar ratio:
2 moles KMnO4 / 5 moles SO3^2- = x moles KMnO4 / 1 mole SO3^2-
Simplifying the proportion:
2/5 = x/1
Cross-multiplying:
2 = 5x
Solving for x:
x = 2/5
Therefore, the number of moles of KMnO4 needed to react with one mole of sulphite ion in acidic solution is 2/5 moles.
Chapter doubts & questions for Mole Concept, Volumetric & Redox - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Mole Concept, Volumetric & Redox - Topicwise Question Bank for IIT JAM/CSIR/GATE Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup